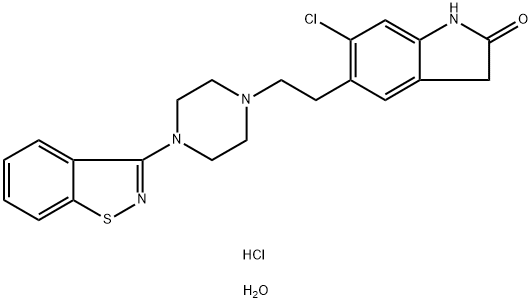
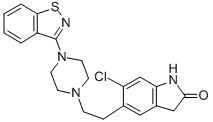
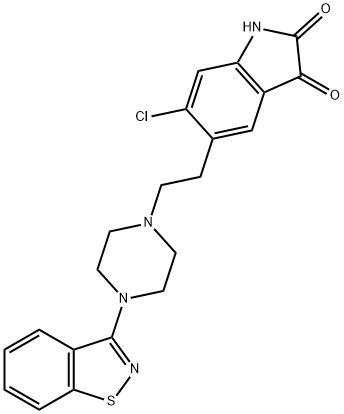
Ziprasidone hydrochloride monohydrate
Synonym(s):5-[2-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one hydrochloride hydrate;5-[2-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one hydrochloride monohydrate;-01;Geodon;Ziprasidone hydrochloride monohydrate
- CAS NO.:138982-67-9
- Empirical Formula: C21H24Cl2N4O2S
- Molecular Weight: 467.41
- MDL number: MFCD06795476
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-12 20:55:48
What is Ziprasidone hydrochloride monohydrate?
Chemical properties
Pale Tan Powder
Originator
Geodon,Pfizer,USA
The Uses of Ziprasidone hydrochloride monohydrate
Combined serotonin (5HT2) and dopamine (D2) receptor antagonist. Used as an antipsychotic.
The Uses of Ziprasidone hydrochloride monohydrate
antipsychotic;Dopamine D2/serotonin 5-HT2 antagonist
The Uses of Ziprasidone hydrochloride monohydrate
Ziprasidone Hydrochloride is the hydrochloride salt of a benzisothiazolylpiperazine analog structurally related to the atypical antipsychotic drug tiospirone that antagonizes both central serotonin 5-HT2A (Ki = 0.42 nM) and dopamine D2 (Ki = 4.8 nM) receptors. It is also a potent agonist at 5-HT1A receptors (Ki = 3.4 nM), increasing cortical dopamine release which may offset negative effects associated with dopamine D2 antagonism, and an inverse agonist at 5-HT1B and 5-HT1D receptors (pKis = 8.8 and 8.6, respectively).[Cayman Chemical]
What are the applications of Application
Ziprasidone hydrochloride monohydrate is an SR-2A and D2DR inhibitor
Definition
ChEBI: The hydrochloride hydrate salt of ziprasidone.
Manufacturing Process
Preparation of 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-chloro-
1,3-dihydro-2H-indol-2-one
A 20-gallon glass lined tank, under a nitrogen atmosphere, was charged with
33.5 liters of water and 9.4 kilograms (kg) of sodium carbonate (dense, 89.1
moles, 3.4 eq.). The resulting mixture was stirred to give a solution. To the
solution 6.4 kg of 2-chloroethyl-6-chloro-oxindole (27.8 moles, 1.06 eq.) was
charged, followed by 6.7 kg of 3-piperazinyl-1,2-benzisothiazole hydrochloride
(26.2 moles, 1.0 eq.). This was stirred and heated to reflux (100°C). After 11
hours the reaction was sampled for high pressure liquid chromatography
(HPLC) assay. The reflux was continued for another 2 hours then the reaction
was cooled to 25°C and the slurry stirred for 1 hour. The product was
observed and found to be essentially free from lumps and gummy matter. The
product was collected by filtration. A 14 liter water was added to the tank and
cooled to 12°C and then used to wash the product. The cake was pulled as
dry as possible, and the product was returned to the tank along with 40 liters
of isopropyl alcohol (IPO). This was cooled and then stirred for 2 hours and
the product was collected by filtration. The cake was washed with 13.4 liters
of fresh IPO, then dried under vacuum at 30° to 40°C. After drying, 17.3 kg
of the title compound was obtained. This was in excess of the theoretical
weight yield due to some residual carbonate in the crude product.
Recrystallization of 5-[2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl]-6-
chloro-1,3-dihydro-2H-indol-2-one
To a clean and dry 100-gallon glass lined tank was charged 9.0 kg of the
material obtained above and 86 gallons of tetrahydrofuran (THF). The slurry
was heated to reflux and held for 1 hour. The hazy solution was then filtered
through a 14" sparkler precoated with filter aid and backed with a Fulflo filter
to a clean, dry, and "spec free" glass-lined tank on a lower level. The batch
was concentrated by vacuum distillation. Another 8.3 kg of the material
obtained in above was dissolved in 83 gallons of THF in the upper tank. This
was filtered to the lower tank. The tank lines and sparkler were rinsed with 10
gallons of THF. The batch was concentrated to about 22 gallons, then cooled
to 5°C and stirred for 1 hour. The product was collected by filtration. Then 20
gallons of fresh IPO were cooled in the tank and used to rinse the product
cake. The product was collected and dried under vacuum at 45°C; yielding
9.05 kg of product (83.8% yield for the coupling and recrystallization. The
product matched the spectra of a standard NMR and showed the correct
retention time by HPLC with 99.7% assay. Another way for preparation of 5-(2-(4-(1,2-benzisothiazol-3-yl)-piperazinyl)ethyl)-6-chloro-1,3-dihydro-2-H-
indol-2-one.
A clean and dry 20-gallon glass lined tank was charged with 19 L of water and
4.44 kg of sodium carbonate, after the carbonate had dissolved 4.29 kg (17.5
moles) of 5-(2-chloroethyl)-6-chloro-oxindole and 3.62 kg (16.5 moles) of 1-
(1,2-benzisothiazol-3-yl)piperazine were added. The aqueous slurry was
heated to reflux and the temperature maintained for 14 hours. When the
reaction was complete the solution was cooled to 20°C and filtered. The wet
product was reslurried in 23 L of isopropyl alcohol at room temperature for 2
hours. The product was collected by filtration on 2 large Buchner funnels, each
was washed with 3.4 L of fresh isopropyl alcohol. The product was vacuum
dried at 30° to 40°C. until no isopropyl alcohol remained, giving 5.89 kg
(86.4% yield) of the desired free base which matched a standard sample by
high performance liquid chromatography (HPLC).
A clean and dry 20-gallon reactor was charged with 17.4 gallons of deionized
water and 4.44 L of concentrated hydrochloric acid, to give a 0.77 M solution.
To the solution was added 4.44 kg of the anhydrous Ziprasidone free base. The slurry was warmed to 65°C and held for 18 hours. The slurry
was cooled to room temperature. The product was filtered and washed with
2x5-gallon portions of deionized water, and then air dried at 50°C for 30
hours. The dried product contained 4.4% water and the x-ray diffraction
method confirmed that the desired product was obtained.
brand name
Geodon (Pfizer).
Therapeutic Function
H-Indol-2-one, 5-(2-(4-(1,2-benzisothiazol-3-yl)-1- piperazinyl)ethyl)-6-chloro-1,3-dihydro-, monohydrochloride monohydrate
Biological Activity
Atypical antipsychotic that displays combined serotonin and dopamine receptor antagonism. Displays high affinity at 5-HT 2A receptors with a 5-HT 2A /D 2 affinity ratio greater than any other clinically available atypical antipsychotics (pK i values are 9.38, 8.88, 8.69, 8.47, 8.32, 8.14, 7.98, 7.49, 7.33 and 6.28 for 5-HT 2A , 5-HT 2C , 5-HT 1D , 5-HT 1A , D 2 , D 3 , α 1 , D 4 , H 1 and D 1 receptors respectively).
Biochem/physiol Actions
Ziprasidone is an atypical antipsychotic; FDA approved for the treatment of schizophrenia.
Biochem/physiol Actions
Ziprasidone (CP-88059) hydrochloride monohydrate is an orally active combined 5-HT and dopamine receptor antagonist. Ziprasidone hydrochloride monohydrate has affinities for Rat D2 (Ki=4.8 nM), 5-HT2A (Ki=0.42 nM) and 5-HT1A (Ki=3.4 nM).
in vitro
Ziprasidone hydrochloride monohydrate (0-500 nM, 150 seconds) blocks wild-type hERG current.
in vivo
Ziprasidone hydrochloride monohydrate (oral gavage; 20 mg/kg; once daily; 7 weeks) results in weight loss, low level physical activity, high resting energy expenditure and greater capacity for thermogenesis when subjected to cold.
storage
Room temperature
Properties of Ziprasidone hydrochloride monohydrate
| Melting point: | 300°C |
| storage temp. | 2-8°C |
| solubility | DMSO: >10mg/mL |
| form | solid |
| color | Light Orange |
| Merck | 14,10171 |
| CAS DataBase Reference | 138982-67-9(CAS DataBase Reference) |
Safety information for Ziprasidone hydrochloride monohydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Ziprasidone hydrochloride monohydrate
New Products
4-Piperidinemethanol Ethyl 2,4-Dihydroxy-6-methylnicotinate Ethyl isonicotinate 3-pyridine methanol N-Methyl 4-chloro-pyridine-2-carboxamide 2-Fluoro-6-iodobenzoic acid 2-((2,6-difluorobenzyl)(ethoxycarbonyl)amino)-4-((dimethylamino)methyl)-5-(4-nitrophenyl)thiophene-3-carboxylic acid Ethyl2-oxo-2,3,9,10-tetrahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(7H)-carboxylate Elinzanetant tert-butyl 2-(4-amino-6-chloropyrimidin-5-yloxy)ethylmethylcarbamate Phenylazomalononitrile 5,6 Dimethoxy-1-indanone 3-Iodophenylacetic acid 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II Bromide Variamine Blue B Diazonium salt N N N'Trimethyl ethylenediamineRelated products of tetrahydrofuran

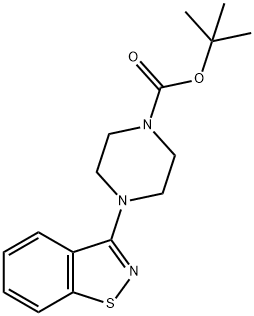
![3-[4-[2-(2,5-Dichloro-4-nitrophenyl)ethyl]-1-piperazinyl]-1,2-benzisothiazole](https://img.chemicalbook.in/CAS/GIF/160384-38-3.gif)
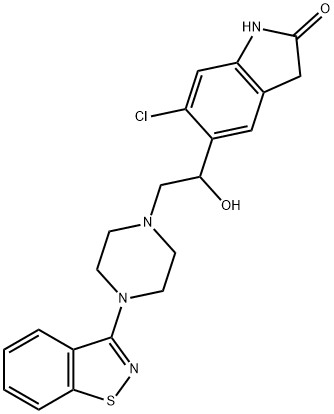
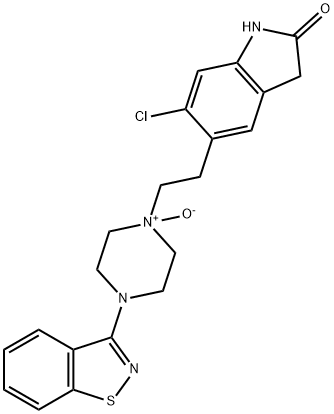
You may like
-
 138982-67-9 Ziprasidone Hydrochloride 98%View Details
138982-67-9 Ziprasidone Hydrochloride 98%View Details
138982-67-9 -
 Ziprasidone hydrochloride monohydrate 99%View Details
Ziprasidone hydrochloride monohydrate 99%View Details
138982-67-9 -
 Ziprasidone Hydrochloride monohydrate 138982-67-9 99%View Details
Ziprasidone Hydrochloride monohydrate 138982-67-9 99%View Details
138982-67-9 -
 138982-67-9 Ziprasidone Hydrochloride monohydrate 98%View Details
138982-67-9 Ziprasidone Hydrochloride monohydrate 98%View Details
138982-67-9 -
 Ziprasidone Hydrochloride Monohydrate CAS 138982-67-9View Details
Ziprasidone Hydrochloride Monohydrate CAS 138982-67-9View Details
138982-67-9 -
 Ziprasidone hydrochloride CAS 138982-67-9View Details
Ziprasidone hydrochloride CAS 138982-67-9View Details
138982-67-9 -
 5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 -
 4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
79307-93-0
