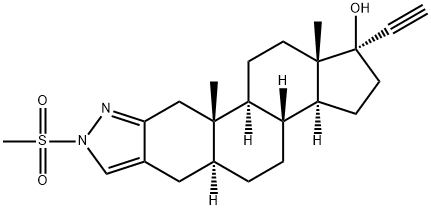
Zanoterone
- CAS NO.:107000-34-0
- Empirical Formula: C23H32N2O3S
- Molecular Weight: 416.58
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is Zanoterone?
Originator
Zanoterone ,Sterling Drug (Sanofi-Aventis)
The Uses of Zanoterone
Anti-androgen.
Definition
ChEBI: Zanoterone is a steroid. It derives from a hydride of an estrane.
Manufacturing Process
1). A solution of methanesulfonylhydrazide (0.0303 mole) in tetrahydrofuran
(70 ml, 30 ml for rinsing) was added with stirring to a solution of
(2α,5α,17α)-2-(diethoxymethyl)-17-[(trifluororacetyl)oxy]pregn-20-yn-3-one
(0.025 mole) in tetrahydrofuran (190 ml). The mixture was allowed to stand
for 3 days at room temperature, heated under reflux for 4 h and evaporated.
The residue was purified by crystallization and recrystallization from methanol,
affording (5α,17α)-1'-(methylsulfonyl)-1'H-pregn-20-yno[3,2-c]pyrazol-17-ol
trifluoroacetate (ester), 69% yield, MP: 166°-168°C.
The above ester (10.24 g, 0.0200 mole) in 100 ml of a solution prepared from
chloroform (210 ml), ethanol (100 ml) and concentrated aqueous ammonia
(10 ml) was allowed to stand at room temperature for 2 h, diluted with
chloroform (250 ml), and washed with dilute hydrochloric acid (2 N, 250 ml).
The chloroform layer was dried and removed of chloroform under vacuum. A
solution of the residue in dichloromethane (95 ml) and ether (5 ml) was
passed through silica gel (50 g) using more dichloromethane-ether (19:1, 600
ml). Evaporation of the solvent and recrystallization of the residue from
acetonitrile afforded (5α,17α)-1'-(methylsulfonyl)-1'H-pregn-20-yno[3,2-
c]pyrazol-17-ol (7.07 g, 85% yield, MP: 202°-203°C). It may be also made
another way.
2). A solution of methanesulfonylhydrazide (82.5 g, 0.75 mole) in acetic acid
(100 ml) was added with stirring over 5 min to a mixture of (5α,17α)-2-
(acetoxymethylene)-17-hydroxypregn-20-yn-3-one (197 g, 0.51 mole) and
acetic acid (1 L). The mixture was stirred for 1 h at room temperature,
forming a deep yellow solution, which was poured with vigorous stirring into
ice-water (6 L). The resulting solid was collected by filtration, washed twice
with water (500 ml each time), pressed dry, washed twice again with water
(500 ml each time), dried (245 g), recrystallized, first from acetonitrile (2.5
volumes) and then from methanol (6.6 volumes), dried, ground, and redried,
affording (5α,17α)-1'-(methylsulfonyl)-1'-H-pregn-20-yno[3,2-c]pyrazol-17-ol
(137.8 g, 65% yield, MP: 194°-196°C).
Therapeutic Function
Antiandrogen
Properties of Zanoterone
| Melting point: | 200-202 °C(Solv: acetonitrile (75-05-8)) |
| Boiling point: | 564.0±60.0 °C(Predicted) |
| Density | 1.36±0.1 g/cm3(Predicted) |
| pka | 13.12±0.60(Predicted) |
Safety information for Zanoterone
Computed Descriptors for Zanoterone
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1
![17b-Hydroxy-5a-androstano[3,2-c]pyrazole](https://img.chemicalbook.in/CAS/GIF/28032-00-0.gif)