Zaldaride
- CAS NO.:109826-26-8
- Empirical Formula: C26H28N4O2
- Molecular Weight: 428.533
- MDL number: MFCD00865523
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
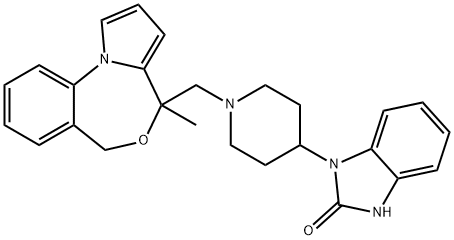
What is Zaldaride?
Originator
Zaldaride maleate,Ciba-Geigy (Novartis)
Definition
ChEBI: 3-[1-[(4-methyl-6H-pyrrolo[1,2-a][4,1]benzoxazepin-4-yl)methyl]-4-piperidinyl]-1H-benzimidazol-2-one is a member of benzimidazoles.
Manufacturing Process
A mixture of 500 g of methyl anthranilate and 438 g of 2,5-
dimethoxytetrahydrofuran in 670 ml of glacial acetic acid is heated at reflux
temperature for 1.5 hours. The acetic acid is then evaporated under reduced
pressure and the residue is distilled to yield methyl 2-(1H-pyrrol-1-
yl)benzoate, b.p. 109°C/0.1 mm Hg.
To a suspension of 117 g of lithium aluminum hydride in 2 L of anhydrous
ether (under an inert atmosphere) is added dropwise a solution of 428 g of
methyl 2-(pyrrol-1-yl)benzoate in 1.5 L of ether over a period of 4 hours. The
reaction mixture is then heated at reflux temperature for an additional 4
hours and then allowed to cool to room temperature. After cooling in an ice-
bath, the excess lithium aluminum hydride is destroyed by the dropwise
addition of 117 ml of water over 1 hour, followed by dropwise addition of 117
ml of 15% sodium hydroxide and subsequent addition of 351 ml of water over
a 30 minute period. The resultant granular solid is separated by filtration, the
ether layer is then dried over magnesium sulfate and the solvent evaporated
under reduced pressure to yield 2-(pyrrol-1-yl)benzyl alcohol which may be
further purified by distillation in vacuum; BP: 110°-114°C/0.1 mm Hg.
To a solution of 34.6 g of 2-(pyrrol-1-yl)benzyl alcohol in 300 ml of anhydrous
tetrahydrofuran and 32 ml of tetramethylethylene diamine is added 183 ml of
a 2.4 molar solution of n-butyl lithium in such a manner that the internal
temperature of the reaction is maintained below 30°C. On completion of the
addition, the reaction mixture is stirred at room temperature for 3 hours. The
reaction mixture is then cooled to -70°C by means of a dry-ice/acetone bath,
and 24 ml of ethyl pyruvate is added to the mixture over 1 minute. The
reaction is then allowed to warm to room temperature and stirred overnight
(18 hours). The reaction is then poured into an ice-water/ether mixture and
the organic phase separated, dried over magnesium sulfate and the solvent
evaporated under reduced pressure to yield ethyl-4-methyl-4H,6H-
pyrrolo[1,2-a][4,1]benzoxazepine-4-carboxylate, MP: 94°-96°C, which may be
recrystallized from a mixture of ether-hexane (1:1).
The mixture of 41 g thereof, 180 ml of 3 N sodium hydroxide and 150 ml of
ethanol is heated at reflux temperature for 6 hours. The ethanol is then
removed by evaporation under reduced pressure and the aqueous solution is
acidified to pH 5 with 6 N HCl. The resulting product, 4-methyl-4H,6H-
pyrrolo[1,2-a][4,1]benzoxazepine-4-carboxylic acid, MP: 182°-183°C, is
collected by filtration, and may be recrystallized from aqueous ethanol.
To a solution of 7.5 g thereof, in 300 ml of tetrahydrofuran is added 5 g of
1,1'-carbonyldiimidazole and the resultant mixture stirred at room
temperature for 1 hour. To this mixture is added 5 g of 1,3-dihydro-1-(4-
piperidyl)-2H-benzimidazol-2-one, and the reaction is heated at reflux
temperature for 48 hours. After cooling to room temperature, the reaction
mixture is poured into 150 ml of ice-water and extracted into 150 ml of
methylene chloride. The organic extracts are washed successively with 150 ml
of sodium carbonate solution, 150 ml of water and 150 ml of dilute
hydrochloric acid, then dried over magnesium sulfate, filtered, and the solvent
is evaporated under reduced pressure to yield 1,3-dihydro-1-{1-[(4-methyl-
4H,6H-pyrrolo[1,2-a][4,1]benzoxazepin-4-yl)carbonyl]-4-piperidinyl}-2H-
benzimidazol-2-one, m.p. 208°-210°C.
To a suspension of 3.0 g of 1,3-dihydro-1-{1-[(4-methyl-4H,6H-pyrrolo[1,2-
a][4,1]benzoxazepin-4-yl)carbonyl]-4-piperidinyl}-2H-benzimidazol-2-one in
120 ml of tetrahydrofuran is added 700 mg of lithium aluminum hydride. The
reaction mixture is then heated at reflux temperature for 6 hours followed by
stirring at room temperature for 18 hours. The reaction mixture is diluted with
150 ml of ether and the excess lithium aluminum hydride destroyed in the
usual manner described below. The resulting granular precipitate is removed
by filtration. The organic phase is washed with 150 ml of dilute sodium
hydroxide and then 150 ml of brine. The organic extracts are then dried over
magnesium sulfate, filtered and solvent evaporated under reduced pressure to
yield 1,3-dihydro-1-{1-[(4-methyl-4H,6H-pyrrolo[1,2-a][4,1]-benzoxazepin-4-
yl)-methyl]-4-piperidinyl}-2H-benzimidazol-2-one, MP: 199°-201°C. This is
then converted into its maleate salt by dissolving separately the free base and
a molar equivalent amount of maleic acid in acetone, and combining the
solutions. The 1:1 maleate salt crystallizes upon standing, has MP: 183°-
185°C.
The 1:1 fumarate salt prepared in a similar manner has a melting point of
125°-127°C.
Therapeutic Function
Antidiarrheal
Properties of Zaldaride
| Melting point: | 173-175° as hydrate |
| Density | 1.31±0.1 g/cm3(Predicted) |
| pka | 12.04±0.30(Predicted) |
Safety information for Zaldaride
Computed Descriptors for Zaldaride
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![[2-(1H-PYRROL-1-YL)PHENYL]METHANOL](https://img.chemicalbook.in/CAS/GIF/61034-86-4.gif)

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4