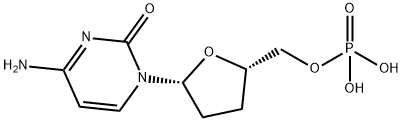
Zalcitabine
Synonym(s):2′,3′-Dideoxycytidine;ddC
- CAS NO.:7481-89-2
- Empirical Formula: C9H13N3O3
- Molecular Weight: 211.22
- MDL number: MFCD00012188
- EINECS: 620-762-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
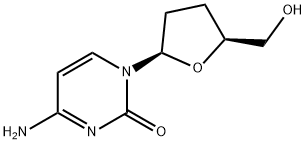
What is Zalcitabine?
Absorption
Bioavailability is over 80% following oral administration.
Toxicity
Acute overdose: Inadvertent pediatric overdoses have occurred with doses up to 1.5 mg/kg zalcitabine. Chronic overdose: in an initial dose-finding study in which zalcitabine was administered at doses 25 times (0.25 mg/kg every 8 hours) the currently recommended dose, one patient discontinued zalcitabine after 1? weeks of treatment subsequent to the development of a rash and fever.
Description
Zalcitabine is an orally active dideoxynucleoside andog for combination use with zidovudine in advanced HIV infection and also as monotherapy for AIDS patients who cannot tolerate or have not responded to zidovudine. It has a similar mechanism of action (inhibition of reverse transcriptase) to didanosine. Like didanosine, its side effect profile includes peripheral neuropathy. Unlike zidovudine, zalcitabine does not cause bone marrow suppression.
Description
Zalcitabine is an analog of pyrimidine derived from deoxycytidine with the replacement of the hydroxyl group in position 3’ with a hydrogen. In cells, it is phosphorylated to the active triphosphate form, ddCTP, which acts as a substrate for HIV reverse transcriptase (Ki = 51 nM). It incorporates into viral DNA where it terminates chain elongation when the missing hydroxyl group is encountered. Zalcitabine was the third antiretroviral approved by the FDA for treatment of HIV infection and AIDS.
Chemical properties
White to Off-White Cyrstalline Powder
Originator
National Cancer Institute (NIH) (U.S.A.)
The Uses of Zalcitabine
A pyrimidine nucleoside analogue with antiviral activity
The Uses of Zalcitabine
ammonia detoxicant, diagnostic aid
The Uses of Zalcitabine
A pyrimidine nucleoside analogue with antiviral activity.
Background
A dideoxynucleoside compound in which the 3'-hydroxyl group on the sugar moiety has been replaced by a hydrogen. This modification prevents the formation of 5' to 3' phosphodiester linkages, which are needed for the elongation of DNA chains, thus resulting in the termination of viral DNA growth. The compound is a potent inhibitor of HIV replication at low concentrations, acting as a chain-terminator of viral DNA by binding to reverse transcriptase. Its principal toxic side effect is axonal degeneration resulting in peripheral neuropathy.
What are the applications of Application
2′,3′-Dideoxycytidine is an antiviral pyrimidine nucleoside analogue effective against HIV replication
Indications
For the treatment of Human immunovirus (HIV) infections in conjunction with other antivirals.
Definition
ChEBI: A pyrimidine 2',3'-dideoxyribonucleoside compound having cytosine as the nucleobase.
Indications
Zalcitabine (ddC, Hivid) is a cytidine analogue active against HIV-1, HIV-2, and hepatitis B virus. It is used for the treatment of HIV infection in adults and asymptomatic children as part of a multidrug regimen. It may be less effective than the other nucleoside inhibitors and is used less frequently.
Manufacturing Process
Bromoacetylation of N-acetylcytidine with 2-acetoxy-2-methylpropanoyl
bromide
A 5 L three-nicked, round-bottomed flask equipped with a mechanical stirrer,
thermometer, nitrogen inlet tube, and additional funnel was charge with 142.6
g (0.5 mole) of N-acetylcytidine, and 1.25 L of acetonitrile. The suspension
was stirred under nitrogen, cooled to 5°C (ice-bath), and treated dropwise
(during 20 min) with 225 ml of 2-acetoxy-2-methylpropanoyl bromide (AIBB)
during 30 minutes. At the completion of the addition, a homogeneous solution
resulted. It was stirred at room temperature overnight (the reaction was
complete within 3 hr), cooled to 5°C, and diluted with 1.25 L of ethyl acetate.
After recooling to 5°C, 2.0 L of saturated sodium bicarbonate was added. The
mixture was stirred for 5 minutes, the organic phase was separated, and the
aqueous phase was back-extracted with 500 ml of ethyl acetate. The
combined organic extracts were washed with 1 L of saturated brine, dried
(MgSO 4 ), and evaporated to give a gum. Final drying at 40°C (1 mm) for 1 hrgave 264.7 g (102%) of a white solid. High pressure liquid chromatographic
analysis gave the following results (major peaks only): 40% of [2R-
[2α,3β,4α,5α(S*)]]-N-[1-[3-(acetyloxy)-5-[(2-(acetyloxy)-1-oxopropoxy]
methyl]-4-bromotetrahydro-2-furanyl]-1,2-dihydro-2-oxo-4-pyrimidinyl]
acetamide (a) and 24% of its regioisomer (b).
Preparation of [2R-[2α,3β,4α,5α(S*)]]-N-[1-[3-(acetyloxy)-5-[(2-(acetyloxy)-
1-oxopropoxy]methyl]-4-bromotetrahydro-2-furanyl]-1,2-dihydro-2-oxo-4-
pyrimidinyl]acetamide (a) and its regioisomer (b).
A 1-L, three-necked, round-bottomed flask equipped with a mechanical stirrer
and argon inlet was charged with 28.52 g of N-acetylcytidine in 250 ml of
acetonitrile. The mixture was cooled to 10°C and treated with 48.75 g of (S)-
(-)-2-acetoxypropionyl bromide during 15 minutes. It was stirred at room
temperature overnight, cooled to 10°C, treated with 400 ml of cold (0°C)
saturated sodium bicarbonate, and extracted with 250 ml of ethyl acetate. The
extract was washed with 200 ml of saturated brine, dried (MgSO 4 ) and
evaporated to give 45.45 g of a white foam. Reversed phase chromatography
(C 18 column) with 40% methanol in water gave a pure sample of (a).
Zinc-copper couple was prepared by the next way:
A 12 L three-necked, round-bottomed flask equipped with a mechanical stirrer
was charged with 4.50 kg of zinc dust. The zinc dust was washed with 3.75 L
of 3% aqueous hydrochloric acid by stirring for 3 to 5 minutes. The
hydrochloric acid was decanted from the solid. This cycle was repeated with
3x3.75 L of 3% hydrochloric acid. The reaction was slightly exothermic and
the volume of the zinc dust increased to double its original volume. The zinc
dust was then washed with 4x3.0 L of deionized water to remove any residual
hydrochloric acid. After all the water was decanted, the spongy zinc layer was
treated with a solution made by dissolving 240.0 g of cupric sulfate dihydrate
in 7.5 L of deionized water. The suspension was stirred rapidly as the solution
was added. The aquamarine color of the cupric sulfate solution was removed
almost immediately and the zinc suspension changed in color from gray to
black. The near colorless aqueous layer was decanted and the solid was
washed with 4x3.0 L of deionized water. The suspension of zinc-copper couple
was filtered through a piece of Whatman No. 1 filter paper, then washed with
4x30 L ethanol and 3x3.0 L of ether. The solid was carefully dried at 25°C and
140 mm overnight to remove ether, then for 3 hr at 130°-140°C (0.5 mm).
The solid was cooled to room temperature under vacuum and was stored
under argon in amber bottles. The procedure yielded 3.84 kg of zinc-copper
couple.
Preparation of [2R-[2α,5α(S*)]]-N-[1-[5-[[2-(acetyloxy)-1-oxopropoxy]
methyl]-2-5-dihydro-2-furanyl]-1,2-dihydro-2-oxo-4-pyrimidinyl]acetamide.
A total of 1.47 g of a mixture of bromoacetates in acetonitril was reduced with
800 mg of zinc-copper couple. The mixture was stirred under argon at room
temperature overnight. The mixture was deoxygenated by evacuation followed
by filling the reaction vessel with argon (oxygen-free nitrogen may be used);
this procedure was repeated three times. It was filtered over Celite, the flask
was rinsed out with of acetonitrile, and the rinse was used to wash the Celite.
The combined filtrate and washing were evaporated (40°C), and the residue
was dissolved in of methylene chloride. This was added to a previously prepared solution of ethylenediaminetetraacetic acid disodium salt dihydrate
(Fluka) in deionized water containing of sodium bicarbonate. The mixture was
stirred vigorously for 1.5 hr, and filtered over Celite, which was washed with
methylene chloride. The organic phase was separated and the aqueous phase
was re-extracted with of methylene chloride. The combined organic was
washed with of saturated sodium bicarbonate, which was back-extracted with
of methylene chloride. The combined organic was dried (MgSO 4 ), filtered, and
concentrated. To this was added of acetic anhydride followed by 40 g of poly-
4-vinylpyridine, and the mixture was stirred under nitrogen for 3 hr. It was
filtered over Celite, which was washed with methylene chloride. The combined
filtrate and washing were evaporated, toluene was added, and the mixture
was evaporated again, ether was added with vigorous stirring for 15 minutes.
The mixture was filtered (some scraping of the flask was necessary) and
washed with ether to give 570 mg of after crystallization from hot
tetrahydrofuran, melting point 125°C; [α] D 25 +119.04°(c=0.25, CHCl 3 ).
Preparation of [2R-[2α,5α(S*)]]-N-[1-[5-[[2-(acetyloxy)-1-oxopropoxy]
methyl]tetrahydro-2-furanyl]-1,2-dihydro-2-oxo-4-pyrimidinyl]acetamide.
A solution of 720 mg of 2R-[2α,5α(S*)]]-N-[1-[5-[[2-(acetyloxy)-1-
oxopropoxy]methyl]-2-5-dihydro-2-furanyl]-1,2-dihydro-2-oxo-4-
pyrimidinyl]acetamide set forth in 10 ml L of methanol and 10 ml of
tetrahydrofuran was hydrogenated over 200 mg of 10% palladium on charcoal
at room temperature and atmospheric pressure until hydrogen uptake ceased
(10 ml). The mixture was filtered over Celite and the filtrate was evaporated
to give a gum. Chromatography on 10 g of silica (70-230 mesh) with 10%
methanol in methylene chloride, gave 290 mg of the product as a foam,
[α] D 25 +88.43° (C=0.99, CHCl 3 ).
Preparation of 2',3'-dideoxycytidine.
A solution of 20.7 g of [2R-[2α,5α(S*)]]-N-[1-[5-[[2-(acetyloxy)-1-
oxopropoxy]methyl]tetrahydro-2-furanyl]-1,2-dihydro-2-oxo-4-pyrimidinyl]
acetamide in 100 ml of ethanol was treated with 10.0 ml of Triton B (N-
benzyltrimethyl-ammonium hydroxide), and the mixture was stirred at room
temperature overnight. The mixture was concentrated to 20 ml, cooled to
0°C, and the product was collected by filtration. It was washed with 10 ml of
cold ethanol to give 4.48 g of 2',3'-dideoxycytidine, melting point 215°-218°C,
as an white solid.
brand name
Hivid (Roche).
Therapeutic Function
Antiviral, Immunosuppressive
General Description
White crystalline powder. Odorless.
General Description
Zalcitabine, 2',3'-dideoxycytidine or ddCyd, is an analog ofcytosine that demonstrates activity against HIV-1 and HIV-2,including strains resistant to AZT. The potency (in peripheralblood mononuclear cells) is similar to that of AZT, but thedrug is more active in populations of monocytes andmacrophages as well as in resting cells.
The oral bioavailability of zalcitabine is over 80% in adultsand less in children.The major dose-limiting side effect isperipheral neuropathy, characterized by pain, paresthesias,and hypesthesia, beginning in the distal lower extremities.These side effects are typically evident after several months oftherapy with zalcitabine. A potentially fatal pancreatitis is anothertoxic effect of treatment with ddC. The drug has beenapproved for the treatment of HIV infection in adults with advanceddisease who are intolerant to AZT or who have diseaseprogression while receiving AZT. ddC is combined with AZTfor the treatment of advanced HIV infection.
Air & Water Reactions
Water soluble.
Reactivity Profile
Zalcitabine may be sensitive to prolonged exposure to light.
Fire Hazard
Flash point data for Zalcitabine are not available; however, Zalcitabine is probably combustible.
Pharmacokinetics
Zalcitabine is an analog of 2'-deoxycytidine that is pharmacologically related to but structurally different from other nucleotide reverse transcriptase inhibitors (NRTIs). Zalcitabine inhibits the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA.
Pharmacology
Peripheral neuropathy occurs in up to 50% of patients taking zalcitabine. Stomatitis, esophageal ulceration, hepatotoxicity, rash, and pancreatitis may occur. Zalcitabine should be used with caution in individuals with a history of pancreatitis, liver disease, or alcohol abuse. Dosage adjustment is necessary for individuals with renal impairment. Zalcitabine should not be used in combination with didanosine, lamivudine, or stavudine.
Pharmacokinetics
Zalcitabine (ddC) is a useful alternate drug to ZDV and is given in combination with ZDV when CD4 cell counts fall to less than 300 cells/mm3 . Monotherapy with ddC is more active than ZDV. Its oral bioavailability is 87%, and its plasma half-life is approximately 1 hour. In low doses (0.005 mg/kg every 4 hours), ddC produces sustained decrease in p24 antigen level and increase in CD4 cell counts. The CSF fluid/plasma ratio of ddC is 0.2. Following oral administration, bioavailability of ddC is less than 80%, which is further reduced when taken with food. The mean maximum plasma concentration of the drug also is reduced from 25.2 to 15.5 ng/mL when the drug was taken with food.
Side Effects
It has side effects, such as stomatitis, rash, fever, malaise, arthritis, and arthralgia.
Metabolism
Zalcitabine is not reported to undergo significant hepatic metabolism; it is mainly phosphorylated intracellularly to zalcitabine triphosphate, the active substrate for HIV-reverse transcriptase. The concentration of zalcitabine triphosphate remains low for quantitative detection and measurement in the plasma following administration of therapeutic doses .
Metabolism
Dideoxyuridine is the major metabolite in urine and feces. The drug penetrates the blood-brain barrier. The major toxicity of ddC is peripheral neuropathy, in which case it should be discontinued. In some cases, pancreatitis occurs when given alone or in combination with ZDV."
Properties of Zalcitabine
| Melting point: | 217-218 °C(lit.) |
| Boiling point: | 350.9°C (rough estimate) |
| alpha | D25 +81° (c = 0.635 in water) |
| Density | 1.2605 (rough estimate) |
| refractive index | 78 ° (C=0.5, H2O) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly), Water (Slightly, Sonicated) |
| form | powder |
| pka | 14.44±0.10(Predicted) |
| color | colorless |
| Water Solubility | 5-10 g/100 mL at 19 ºC |
| Merck | 14,10109 |
| BRN | 654956 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 7481-89-2(CAS DataBase Reference) |
| IARC | 2B (Vol. 76) 2000 |
| EPA Substance Registry System | Cytidine, 2',3'-dideoxy- (7481-89-2) |
Safety information for Zalcitabine
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P281:Use personal protective equipment as required. |
Computed Descriptors for Zalcitabine
| InChIKey | WREGKURFCTUGRC-POYBYMJQSA-N |
Abamectin manufacturer
ANAXLABORATORIES PRIVATE LIMITED
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![10,11-Dihydro-alpha-methyl-10-oxo-dibenzo[b,f]thiepin-2-acetic acid](https://img.chemicalbook.in/CAS/GIF/74711-43-6.gif)


You may like
-
 2,3-Dideoxycytidine (ddC) extrapure CAS 7481-89-2View Details
2,3-Dideoxycytidine (ddC) extrapure CAS 7481-89-2View Details
7481-89-2 -
 2′,3′-Dideoxycytidine CAS 7481-89-2View Details
2′,3′-Dideoxycytidine CAS 7481-89-2View Details
7481-89-2 -
 2′,3′-Dideoxycytidine CAS 7481-89-2View Details
2′,3′-Dideoxycytidine CAS 7481-89-2View Details
7481-89-2 -
 Zalcitabine CAS 7481-89-2View Details
Zalcitabine CAS 7481-89-2View Details
7481-89-2 -
 4-AMINO-1-(5-HYDROXYMETHYL-TETRAHYDRO-FURAN-2-YL)-1H-PYRIMIDIN-2-ONE CAS 7481-89-2View Details
4-AMINO-1-(5-HYDROXYMETHYL-TETRAHYDRO-FURAN-2-YL)-1H-PYRIMIDIN-2-ONE CAS 7481-89-2View Details
7481-89-2 -
 7481-89-2 Dideoxycytidine 98%View Details
7481-89-2 Dideoxycytidine 98%View Details
7481-89-2 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4