Zabicipril
- CAS NO.:83059-56-7
- Empirical Formula: C23H32N2O5
- Molecular Weight: 416.515
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
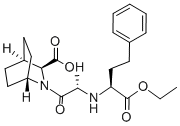
What is Zabicipril?
Originator
Zabicipril ,ZYF Pharm Chemical
Manufacturing Process
The racemic 2-azabicyclo[2.2.1]heptane-3-carboxylic acid was obtained by alkaline hydrolysis (24 h reflux in 4 N NaOH/MeOH 3/1; yield 78%) of the 4- phenyl-2,4-diazatricyclo[5.2.1.0 2,6 ]decane-3,5-dione-(1)- hydantoin obtained according to Ben Ishai. Starting from 2-azabicyclo[2.2.1]heptane-3-carboxylic acid, the (-)-tartaric acid salt of it crystallized from EtOH (yield 87 %) was obtained from this salt by ion-exchange on Dowex 50 WX 8 (H+ form) and elution with 0.3 N NaOH. (yield 98 %) (GLC after esterification with CH 2 N 2 and amidation with (-)-camphanyl chloride). 2-Azabicyclo[2.2.1]heptane-3- carboxylic acid was esterified to benzyl ester with benzyl alcohol in toluene using p-toluenesulfonic acid as catalyst. The second chiral intermediate - 4- phenylbutyric acid ethyl ester; compound with 2-amino-propionic acid (alanine) was prepared according to Kaltenbronn S. It was coupled with the above prepared benzyl ester using dicyclohexylcarbodiimideoxybenztriasole (DCC-HOBT)-Et 3 N in DMF, thus producing the benxyl ester 2-[2-(1- ethoxycarbonyl-3-phenylpropylamino)propionyl]-2-azabicyclo[2.2.1]heptane- 3-carboxylic acid benzyl ester (yield 97 %). The high yield of this coupling is probably due to the high reactivity of the rigid bulky nucleophile 2-[2-(1- ethoxycarbonyl-3-phenylpropylamino)propionyl]-2-azabicyclo[2.2.1]heptane- 3-carboxylic acid benzyl ester and to the low reactivity of the sterically hindered secondary amine intermediate - 4-phenylbutyric acid ethyl ester; compound with 2-amino-propionic acid, so that the formation of by-products (racemates, diketopiperazine from two acylurea by addition of acid on DCC) was not observed (TLC). Benzyl ester was submitted to hydrogenolysis on palladium charcoal in EtOH at room temperature (yield 98%). It crystallized as its t-butylamine salt from ether and finally transformed to more stable hydrochloride - zabicipril (yield 95 %). Careful saponification of t-butylamine salt with 1 N NaOH at room temperature gave crude zabiciprilate, which was purified by ion-exchange on Dowex 50 WX 8 (H + form) and elution with water/pyridine 9:1, then crystallization from 2-propanol (yield 80%). Structure of all described compounds is confirmed with NMR spectrum and X- Ray crystal structure analysis.
Therapeutic Function
Antihypertensive
Safety information for Zabicipril
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

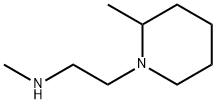

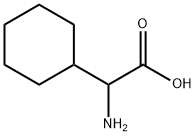
![2-Azabicyclo[2.2.2]octane-3-carboxylicacid,(3S)-(9CI)](https://img.chemicalbook.in/CAS/GIF/109583-12-2.gif)
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
