XENON DIFLUORIDE
Synonym(s):Xenon difluoride
- CAS NO.:13709-36-9
- Empirical Formula: F2Xe
- Molecular Weight: 169.29
- MDL number: MFCD00040538
- EINECS: 237-251-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-12-20 12:09:19
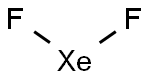
What is XENON DIFLUORIDE?
Description
Xenon difluoride is a white crystalline solid with an unpleasant odor. One of the first compounds to be prepared from "inert" gases, it was synthesized by the low-pressure photochemical reaction of xenon and fluorine by J. L. Weeks, C. L. Chernick, and M. S. Matheson in 1962. The same year, R. Hoppe and co-workers produced it via electrical discharge. XeF2 is a strong fluorinating agent.
Chemical properties
white crystalline solid
The Uses of XENON DIFLUORIDE
Xenon Difluoride is a fluorination agent used in gas chromatography.
What are the applications of Application
Xenon difluoride is a fluorinating agent
General Description
Xenon fluoride may be obtained by interacting elemental xenon and fluorine in the temperature range of 473-523 oC and 5 absolute atmosphere. Xenon difluoride readily interacts with Lewis acid and forms complexes.
Properties of XENON DIFLUORIDE
| Melting point: | 129 °C (lit.) |
| Boiling point: | 114.35°C (estimate) |
| Density | 4.32 g/mL at 25 °C (lit.) |
| vapor pressure | 3.8 mm Hg ( 25 °C) |
| Flash point: | None |
| storage temp. | Store at +2°C to +8°C. |
| solubility | Acetonitrile (Slightly), DMSO (Slightly) |
| form | Crystals |
| color | White |
| Specific Gravity | 4.320 |
| Water Solubility | Decomposes in water. |
| Sensitive | Moisture Sensitive |
| Merck | 14,10072 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability: | Stable. Incompatible with air, moisture. Releases fluorine on decomposition. Contact with combustible material may cause fire. (Traces of xenon tetrafluoride are sometimes found with this material. When exposed to moisture, the tetrafluoride may form the explosive xenon trioxide.) |
| CAS DataBase Reference | 13709-36-9(CAS DataBase Reference) |
| EPA Substance Registry System | Xenon fluoride (XeF2) (13709-36-9) |
Safety information for XENON DIFLUORIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H301:Acute toxicity,oral H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for XENON DIFLUORIDE
| InChIKey | IGELFKKMDLGCJO-UHFFFAOYSA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 Xenon difluoride CAS 13709-36-9View Details
Xenon difluoride CAS 13709-36-9View Details
13709-36-9 -
 Xenon Difluoride CAS 13709-36-9View Details
Xenon Difluoride CAS 13709-36-9View Details
13709-36-9 -
 Xenon Difluoride CAS 13709-36-9View Details
Xenon Difluoride CAS 13709-36-9View Details
13709-36-9 -
 Xenon difluoride CAS 13709-36-9View Details
Xenon difluoride CAS 13709-36-9View Details
13709-36-9 -
 Xenon difluoride CAS 13709-36-9View Details
Xenon difluoride CAS 13709-36-9View Details
13709-36-9 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4