VITAMIN K1
- CAS NO.:12001-79-5
- Empirical Formula: C31H46O2
- Molecular Weight: 450.7
- MDL number: MFCD00001683
- EINECS: 234-408-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
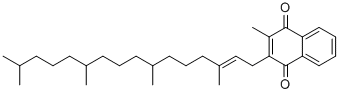
What is VITAMIN K1?
Definition
ChEBI: 2-methyl-3-(3,7,11,15-tetramethylhexadec-2-enyl)naphthalene-1,4-dione is a member of 1,4-naphthoquinones.
Biological Functions
Both vitamins K3 and K4 may produce hyperbilirubinemia and kernicterus in neonates as well as hemolysis in neonates and glucose-6-phosphate–deficient patients. In fact, the only advantage of vitamins K3 and K4 over vitamin K1 is that whereas absorption of vitamin K1 requires the presence of bile, absorption of vitamins K3 and K4 does not, because they are absorbed via a passive process directly from the intestine. This may be a slight advantage for patients with cholestasis or severe pancreatic dysfunction. Only vitamin K1, however, is appropriate therapy for bleeding associated with warfarin and superwarfarin anticoagulation. Vitamin K2 is not used therapeutically.
Mechanism of action
Vitamin K antagonists, such as warfarin, produce their effect on blood coagulation by interfering with the cyclic interconversion of vitamin K and vitamin K 2,3-epoxide. Vitamin K is an essential cofactor necessary for the posttranslational carboxylation of the glutamic acid residues on the N-terminal portions of the specific clotting factors (II, VII, IX, and X) and anticoagulant proteins, such as protein C. This γ-glutamyl carboxylation results in a new amino acid, γ-carboxyglutamate, which through chelation of calcium ions causes the proteins to undergo a conformational change. This change in tertiary structure allows the four vitamin K–dependent clotting factors to become activated and bind to the negatively charged phospholipid membranes during clotting cascade activation.
Pharmacokinetics
The half-life of vitamin K1 is quite short—only 1.7 hours via the intravenous route and 3–5 hours via the oral route. When given orally, vitamin K1 is absorbed directly from the proximal small intestine in an energy-dependent and saturable process that requires the presence of bile salts. These kinetic features argue for administration in divided doses rather than larger, single daily doses. The typical starting point for adults with drug-induced hypoprothrombinemia is 2.5 to 10 mg of vitamin K1 orally, repeating in 12 to 48 hours if needed. In cases of ingestion of long-acting superwarfarin rodenticides (e.g., brodifacoum), therapy may be 125 mg/day for weeks or months. Practically speaking, because vitamin K1 is dispensed as 5-mg tablets, superwarfarin-poisoned patients may require 10 to 30 tablets every 6 hours.
Clinical Use
Vitamin K1 (phytonadione, Mephyton) is the form of vitamin K most often used therapeutically. Vitamin K1 is safe for use in infants, pregnant women, and patients with glucose-6-phosphate deficiency.
Safety Profile
Moderately toxic by subcutaneous route. An experimental teratogen. When heated to decomposition it emits acrid smoke and irritating fumes.
Properties of VITAMIN K1
| Melting point: | −20 °C(lit.) |
| Density | 0.984 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO |
| form | viscous liquid |
| color | Light yellow to yellow |
| CAS DataBase Reference | 12001-79-5 |
| IARC | 3 (Vol. 76) 2000 |
| EPA Substance Registry System | Vitamin K (12001-79-5) |
Safety information for VITAMIN K1
Computed Descriptors for VITAMIN K1
VITAMIN K1 manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 12001-79-5 Vitamin K 98%View Details
12001-79-5 Vitamin K 98%View Details
12001-79-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1