Vinylphosphonic acid
Synonym(s):p -Ethenylphosphonic acid
- CAS NO.:1746-03-8
- Empirical Formula: C2H5O3P
- Molecular Weight: 108.03
- MDL number: MFCD00043866
- EINECS: 217-123-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-22 15:21:50
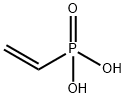
What is Vinylphosphonic acid?
The Uses of Vinylphosphonic acid
Vinylphosphonic acid (VPA) can be used as a monomer unit for the synthesis of poly(vinylphosphonic acid) via free radical polymerization. It is also used to develop copolymers of VPA with acrylonitrile, N-isopropylacrylamide, styrene, vinylpyrrolidone, and acrylic and methacrylic acid. These copolymers find potential application in hydrogels, drug delivery, biomimetic mineralization, and polymer electrolyte membranes in fuel cells.
It can also be used as an organic building block to prepare (E)-styryl phosphonic acid derivatives by reacting with various aryl halides via Pd-catalyzed Heck coupling reaction.
The Uses of Vinylphosphonic acid
VPA based homopolymers and copolymers find usage in corrosion treatment, fuel cells, dental cement, drug delivery, and bio-mimicry.
General Description
Vinylphosphonic acid (VPA) is an organophosphorus compound that is used in the surface treatment of metal substrates. It can be used in the preparation of poly(VPA) by radical polymerization in the presence of initiator systems and chain transfer agents. PVPA tends to have an electrolytic nature, which is useful for a variety of energy based applications.
Flammability and Explosibility
Not classified
Purification Methods
This fireproofing agent, and ingredient for making polymers, is obtained as a syrup on hydrolyzing vinylphosphonyl dichloride with cold H2O and solidifies on prolonged drying over P2O5/KOH. When distilled at 235-240o/0.0006mm, it gives the anhydride (d 1.304, n 1.5874) [Kabachnik & Medvedi Izvest Akad Nauk SSSR, Ser Khim 868 1953, Chem Abstr 54 10834 1960]. It is best kept as the sodium salt (m 350o) which precipitates when a solution of EtOH containing NaOEt (from 2g of Na) is added to vinylphosphonic acid (3.2g), and is recrystallised from EtOH (5.1g, quantitative). The p-anisidinium salt forms mauve prisms m 250o (from EtOH/Et2O). The dimethyl ester, M 136.1, d4 1.1405, nD 1.4330, has b 72.5o/10mm and 197-202o/760mm. [Kabachnik et al. J Gen Chem USSR (Engl Trans) 33 375 1963, Beilstein 4 IV 3568.]
Properties of Vinylphosphonic acid
| Melting point: | 41-45 °C(lit.) |
| Boiling point: | 97-98 °C(Press: 2 Torr) |
| Density | 1.389 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 20℃ |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in water |
| form | liquid |
| pka | 2.11±0.10(Predicted) |
| color | colorless to pale-yellow |
| PH | 1.5 (50g/l, H2O, 30℃) |
| Water Solubility | 100g/L at 20℃ |
| BRN | 1741622 |
| CAS DataBase Reference | 1746-03-8(CAS DataBase Reference) |
| EPA Substance Registry System | Phosphonic acid, ethenyl- (1746-03-8) |
Safety information for Vinylphosphonic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Vinylphosphonic acid
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Vinylphosphonic Acid CAS 1746-03-8View Details
Vinylphosphonic Acid CAS 1746-03-8View Details
1746-03-8 -
 Vinylphosphonic acid CAS 1746-03-8View Details
Vinylphosphonic acid CAS 1746-03-8View Details
1746-03-8 -
 Vinylphosphonic acid CAS 1746-03-8View Details
Vinylphosphonic acid CAS 1746-03-8View Details
1746-03-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
