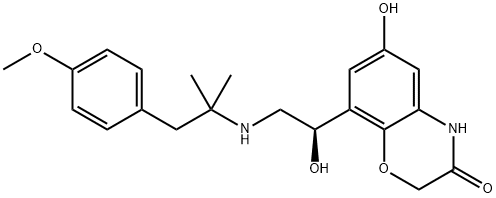
Vilanterol
- CAS NO.:503068-34-6
- Empirical Formula: C24H33Cl2NO5
- Molecular Weight: 486.43
- MDL number: MFCD18782703
- EINECS: 690-631-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-28 17:34:51
What is Vilanterol?
Absorption
Vilanterol plasma levels may not predict therapeutic effects. Following inhaled administration of vilanterol in healthy subjects, Cmax occurred at 5 to 15 minutes. Vilanterol is mostly absorbed from the lung after inhaled doses with negligible contribution from oral absorption. Following repeat dosing of inhaled vilanterol, the steady state was achieved within 14 days with up to 1.7-fold accumulation.
The absolute bioavailability of vilanterol when administered by inhalation was 27.3%, primarily due to absorption of the inhaled
portion of the dose delivered to the lung. Oral bioavailability from the swallowed portion of the dose of vilanterol is low (<2%) due to extensive first-pass metabolism. Systemic exposure (AUC) in patients with COPD was 24% higher than observed in healthy subjects. Systemic exposure (AUC) in patients with asthma was 21% lower than observed in healthy subjects.
Toxicity
In separate embryofetal developmental studies, pregnant rats and rabbits received vilanterol during the period of organogenesis at doses up to approximately 13,000 and 450 times, respectively, the maximum recommended human daily inhaled dose (MRHDID) (on an mcg/m2 basis at maternal inhalation doses up to 33,700 mcg/kg/day in rats and on an AUC basis at maternal inhaled doses up to 5,740 mcg/kg/day in rabbits). No evidence of structural abnormalities was observed at any dose in rats or in rabbits up to approximately 70 times the MRHDID (on an AUC basis at maternal doses up to 591 mcg/kg/day in rabbits). However, fetal skeletal variations were observed in rabbits at approximately 450 times the MRHDID (on an AUC basis at maternal inhaled or subcutaneous doses of 5,740 or 300 mcg/kg/day, respectively). The skeletal variations included decreased or absent ossification in the cervical vertebral centrum and metacarpals.
In a perinatal and postnatal developmental study in rats, dams received vilanterol during late gestation and the lactation periods at doses up to approximately 3,900 times the MRHDID (on an mcg/m2 basis at maternal oral doses up to 10,000 mcg/kg/day). No evidence of effects on offspring development was observed.
The expected signs and symptoms with overdosage of vilanterol are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the signs and symptoms of beta-adrenergic stimulation (e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, muscle cramps, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, insomnia, hyperglycemia, hypokalemia, metabolic acidosis). As with all inhaled sympathomimetic medicines, cardiac arrest, and even death may be associated with an overdose of vilanterol.
In a 2-year carcinogenicity study in mice, vilanterol caused a statistically significant increase in ovarian tubulostromal adenomas in females at an inhaled dose of 29,500 mcg/kg/day (approximately 7,800 times the MRHDID for adults on an AUC basis). No increase in tumors was seen at an inhaled dose of 615 mcg/kg/day (approximately 210 times the MRHDID for adults on an AUC basis).
In a 2-year carcinogenicity study in rats, vilanterol caused statistically significant increases in mesovarian leiomyomas in females and a shortening of the latency of pituitary tumors at inhaled doses greater than or equal to 84.4 mcg/kg/day (greater than or equal to approximately 20 times the MRHDID for adults on an AUC basis). No tumors were seen at an inhaled dose of 10.5 mcg/kg/day (approximately equal to the MRHDID for adults on an AUC basis).
These tumor findings in rodents are similar to those reported previously for other beta-adrenergic agonist drugs. The relevance of these findings to human use is unknown.
Vilanterol tested negative in the following genotoxicity assays: the in vitro Ames assay, in vivo rat bone marrow micronucleus assay, in vivo rat unscheduled DNA synthesis (UDS) assay, and in vitro Syrian hamster embryo (SHE) cell assay. Vilanterol tested equivocal in the in vitro mouse lymphoma assay.
Indications
Vilanterol is approved for use in several combination products such as with fluticasone furoate under the tradename Breo Ellipta, in combination with umeclidinium bromide as Anoro Ellipta, and in combination with both fluticasone furoate and umeclidinium under the tradename Trelegy Ellipta.
Approved by the FDA in 2013, the use of Breo Ellipta is indicated for the long-term, once-daily maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and emphysema, as well as the once-daily maintenance treatment of asthma in patients aged 18 or older with reversible obstructive airways disease. Anoro Ellipta is indicated for the maintenance treatment of patients with COPD, and Trelegy Ellipta is indicated for the maintenance treatment of patients with COPD as well as the maintenance treatment of asthma in patients aged 18 years and older.
Background
Vilanterol is a selective long-acting β2-adrenergic agonist (LABA) with inherent 24-hour activity for the once-daily treatment of COPD and asthma. This is in response to the need for longer-acting β2-adrenergic agonists to overcome poor patient compliance (due to the frequency of dosing regimens or complexities of drug administration). Vilanterol was designed based on the salmeterol molecular scaffold, particularly as a antedrug analog of salmeterol modification by modifying the salmeterol molecule to create homochiral compounds with the (R)-configuration. Vilanterol is 1000 and 400 fold more selective for β2 than β1 and β3 adrenoceptors, respectively, with a faster onset of action than salmeterol. Additionally, vilanterol demonstrated a significantly longer duration of action than salmeterol, with the bronchodilator effect still apparent at 22h. Vilanterol's pharmacological effect is attributable to stimulation of intracellular adenylyl cyclase which catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3',5'-adenosine monophosphate (cAMP). Increases in cyclic AMP are associated with the relaxation of bronchial smooth muscle and inhibition of the release of hypersensitivity mediators from mast cells in the lungs.
Vilanterol is approved for use in several combination products such as with fluticasone furoate under the tradename BREO ELLIPTA, with [umeclidinium bromide] as ANORO ELLIPTA, and with both fluticasone furoate and [umeclidinium bromide] under the trade name TRELEGY ELLIPTA. BREO ELLIPTA is the first vilanterol-containing product to be approved by the FDA in May 2013, followed by ANORO ELLIPTA in December 2013 and TRELEGY ELLIPTA in September 2020. Although all 3 products are approved for the maintenance treatment of chronic obstructive pulmonary disease (COPD), only TRELEGY ELLIPTA and BREO ELLIPTA are approved for maintenance treatments of asthma in patients aged 18 years and older and 5 years and older respectively.
Definition
ChEBI: An dichlorobenzene derivative that is used in the form of its trifenate salt for treatment of chronic obstructive pulmonary disease.
Biological Activity
vilanterol is a novel and selective agonist of β2-ar with a pec50 value of 10.37±0.05 [1].vilanterol is a novel long-acting β2-ar agonist (laba) with 24h activity in development for inhaled once daily treatment. in the radioligand binding studies, vilanterol has shown the binding affinity in the one-affinity site model with pkd values of 9.44±0.07 and 10.82±0.12 in the presence gpp(nh)p and absence gpp(nh)p, respectively. in dissociation studies, vilanterol has been reported to bind from the β2-ar with a dissociation t1/2 value of 3.5 min in the presence of gpp(nh)p. vilanterol has been found to have a good selectivity for β2-ar over the other β-ar receptor subtypes(β1and β3) with pec50 values of 10.37±0.05, 6.98±0.03 and 7.36±0.03, respectively. vilanterol has exhibited at least 1000-fold selectivity over both β1-and β3-ar subtypes [1].
Metabolism
Vilanterol is principally metabolized by cytochrome p450 3A4 (CYP3A4) to a range of metabolites with significantly reduced beta1- and beta2-agonist activity. The major route of metabolism was via O-dealkylation, with up to 78% of the recovered dose eliminated as O-dealkylated metabolites while N-Dealkylation and C-dealkylation were minor pathways, representing 5% of the recovered dose.
References
[1] slack rj1, barrett vj, morrison vs, sturton rg, emmons aj, ford aj, knowles rg.in vitro pharmacological characterization of vilanterol, a novel long-acting β2-adrenoceptor agonist with 24-hour duration of action.j pharmacol exp ther. 2013 jan; 344(1):218-30.
Properties of Vilanterol
| Boiling point: | 646.7±55.0 °C(Predicted) |
| Density | 1?+-.0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | solution in ethanol |
| pka | 9.99±0.20(Predicted) |
| color | Light yellow to yellow |
Safety information for Vilanterol
Computed Descriptors for Vilanterol
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 503068-34-6 Vilanterol 98%View Details
503068-34-6 Vilanterol 98%View Details
503068-34-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8