VERRUCULOGEN
Synonym(s):GPR70;T1R1;TAS1R1;Taste receptor type 1 member 1;TR1
- CAS NO.:12771-72-1
- Empirical Formula: C27H33N3O7
- Molecular Weight: 511.57
- MDL number: MFCD00079645
- EINECS: 634-189-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
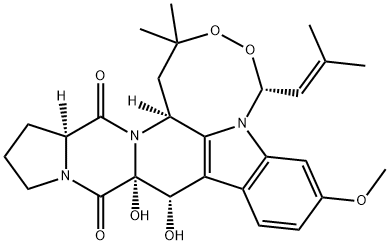
What is VERRUCULOGEN?
The Uses of VERRUCULOGEN
Verruculogen is a tremorgenic mycotoxin, first isolated from Penicillium verruculosum in 1972. The structure was resolved as an indole alkaloid in 1974. Verruculogen is produced by several species of Penicillium and Aspergillus and its presence is a useful taxonomic phenotypic marker. The tremorgenic action of verruculogen is associated with increases in spontaneous glutamate and aspartate release, decreases in GABA levels and, at toxic doses, an increase in the number and decrease in the affinity of DHP receptors in rat cortex. In in vitro guinea pig ileum preparations, verruculogen causes an increase in contractile responses due to electrical field stimulation, attributed to enhancement of acetylcholine from presynaptic nerve terminals. Verruculogen also inhibits Ca2+-activated K+ channels, and is a cell cycle inhibitor blocking division at the M phase.
The Uses of VERRUCULOGEN
Verruculogen is a tremorgenic mycotoxin isolated from species of Penicillium, Aspergillus, and other fungi. It selectively inhibits the activation of Maxi-K potassium channels by charybdotoxin with a K1/2 value of 170 nM. Verruculogen also promotes the release of excitatory neurotransmitters when injected directly into the brain of rats and, at high doses, arrests mouse mammary carcinoma cells in M phase of the cell cycle (MIC = 12.2 μM).[Cayman Chemical]
The Uses of VERRUCULOGEN
Suggested starting dilutions are as follows: ICC/IF: 1:100-1:1000, IHC-P: 1:100-1:1000, WB: 1:500-1:3000. Not yet tested in other applications. Optimal working dilutions should be determined experimentally by the end user.
What are the applications of Application
Verruculogen is a neurotoxin that inhibits the M phase of the mammalian cell cycle
Definition
ChEBI: An organic heterohexacyclic compound that is a mycotoxic indole alkaloid isolated from Penicillium and Aspergillus species.
Biological Activity
verruculogen is a maxi-k potassium channels inhibitor.maxi-k, a potassium channel characterized by their large conductance for potassium ions through cell membranes, is activated by changes in membrane electrical potential and/or by increases in concentration of intracellular calcium ion. maxi-k channels are critical for the regulation of several key physiological processes, such as smooth muscle tone and neuronal excitability.
Biochem/physiol Actions
The protein encoded by this gene is a G protein-coupled receptor and is a component of the heterodimeric amino acid taste receptor T1R1+3. The T1R1+3 receptor responds to L-amino acids but not to D-enantiomers or other compounds. Most amino acids that are perceived as sweet activate T1R1+3, and this activation is strictly dependent on an intact T1R1+3 heterodimer. Multiple transcript variants encoding several different isoforms have been found for this gene. [provided by RefSeq]
in vitro
in a previous study, it was found that aflatrem, paspalitrem a, paspalitrem c, penitrem a, and paspalinine could inhibit the binding of [125i]charybdotoxin to maxi-k channels in bovine aortic smooth muscle sarcolemmal membranes. whereas, verruculogen enhanced toxin binding. despite their different effects on binding of [125i]charybdotoxin to maxi-k channels, all tested compounds including verruculogen were able to potently inhibit maxi-k channels in electrophysiological experiments, and other types of voltage-dependent or ca(2+)-activated k+ channels examined were not affected [1].
in vivo
in an animal study, the mechanism of the genesis of tremor induced by verruculogen was investigated. it was found that atropine, glycine, mephenesin, and penicillamine with proven efficacy in relieving tremor of varied etiology, failed to modify verruculogen induced tremor. oxyaminoacetic acid, which raises the cns levels of gaba, and beta (chlorphenyl) gamma aminobutyric acid, which passes the hematoencephalic barrier, completely or partly relieved tremor and other verruculogen-induced symptoms, depending on the dose of the verruculogen administered. picrotoxin, a gaba antagonist, increased the effect of verruculogen in the tested animals [2].
storage
+4°C
References
[1] knaus, h. g.,mcmanus, o.b.,lee, s.h., et al. tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. biochemistry 33(19), 5819-5828 (1994).
[2] hotujac l, stern p. pharmacological examination of verruculogen induced tremor. acta med iugosl. 1974;28(3):223-9.
Properties of VERRUCULOGEN
| Boiling point: | 738.4±60.0 °C(Predicted) |
| Density | 1.49±0.1 g/cm3(Predicted) |
| Flash point: | 2℃ |
| storage temp. | 2-8°C |
| solubility | Soluble in ethanol;Soluble in methanol;Soluble in DMSO;Soluble in dimethyl formamide |
| form | buffered aqueous solution |
| pka | 10?+-.0.70(Predicted) |
| color | White to off-white |
Safety information for VERRUCULOGEN
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for VERRUCULOGEN
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1