Vecuronium bromide
Synonym(s):1-[(2β,3α,5α,16β,17β)-3,17-Bis(acetyloxy)-2-(1-piperidinyl)androstan-16-yl]-1-methyl-piperidinium bromide;Vecuronium bromide
- CAS NO.:50700-72-6
- Empirical Formula: C34H57BrN2O4
- Molecular Weight: 637.73138
- MDL number: MFCD00867762
- EINECS: 256-723-9
- Update Date: 2025-12-12 11:51:25
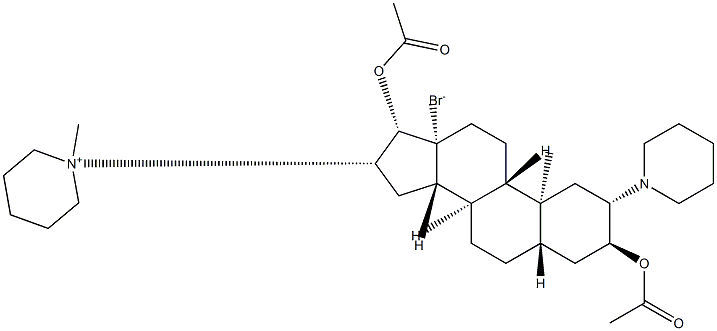
What is Vecuronium bromide?
Description
Vecuronium, 1-[(2β,3α,5α,16β,17β,)-3,17-bis(acetyloxy)-2-(1-piperidinyl) androstan-16-yl]-1-methylpiperidinium bromide (15.1.9), differs from panuronium only in the extent of alkylation. Only the piperidine substituent on C16 of the steroid skeleton is transformed into a quaternary salt.
Description
Vecuronium is a non-depolarizing muscle relaxant derived from the aminosteroid pancuronium and used adjunctively to general anesthesia. It competitively blocks cholinergic receptors at the motor end plate of the neuromuscular junction, inducing temporary paralysis. In humans, it has been shown to reduce muscle twitch tension with an ED50 value of 0.15 mg/kg for a duration of 27 minutes without inducing cardiovascular effects.
Chemical properties
White Solid
The Uses of Vecuronium bromide
An aminosteroid, competitive neuromuscular blocker agent.
The Uses of Vecuronium bromide
alcohol antagonist
What are the applications of Application
Vecuronium bromide is ompetes with acetylcholine and binds to cholinergic receptors
Definition
ChEBI: The organic bromide salt of a 5alpha-androstane compound having 3alpha-acetoxy-, 17beta-acetoxy-, 2beta-piperidinino- and 16beta-N-methylpiperidinium su stituents.
brand name
Norcuron (Organon).
Biological Functions
Vecuronium bromide (Norcuron) is chemically identical to pancuronium except for a tertiary amine in place of a quaternary nitrogen. However, some of the drug will exist as the bisquaternary compound, depending on body pH. Vecuronium has a moderate onset of action (2.4 minutes) and a duration of effect of about 50 minutes. Like pancuronium, it does not block ganglia or vagal neuroeffector junctions, does not release histamine, and is eliminated by urinary excretion.
General Description
Vecuronium bromide, 1-(3 ,17 -dihydroxy-2 -piperidino-5 -androstan-16 -yl)-1-methylpiperidinium bromide diacetate (Norcuron), is themonoquaternary analog of pancuronium bromide. It belongsto the class of nondepolarizing neuromuscular blockingagents and produces effects similar to those of drugs in thisclass. It is unstable in the presence of acids and undergoesgradual hydrolysis of its ester functions in aqueous solution.Aqueous solutions have a pH of about 4.0.
Mechanism of action
Vecuronium bromide is a bisquaternary nitrogen compound that acts by competitively binding to nicotinic cholinergic receptors. The binding of vecuronium decreases the opportunity for acetylcholine to bind to the nicotinic receptor at the postjunctional membrane of the myoneural junction. As a result, depolarization is prevented, calcium ions are not released and muscle contraction does not occur.
Pharmacology
Vecuronium bromide is steroidal agent that was developed in an attempt to reduce the cardiovascular effects of pancuronium. I t is similar in structure to the older drug, differing only in the loss of a methyl group from one quaternary ammonium radical. Thus it is a monoquaternary amine. An intubating dose of 0.1mgkg-1 produces profound neuromuscular block within 3min. This dose produces clinical block for about 30min. Vecuronium rarely produces histamine release, nor does it have any direct cardiovascular effects, although it allows the cardiac effects of other anaesthetic agents, such as bradycardia produced by the opioids, to go unchallenged. Vecuronium is excreted through the kidneys (30%), although to a lesser extent than pancuronium, and undergoes hepatic deacetylation; the deacetylated metabolites have neuromuscular blocking properties. Repeated doses should be used with care in patients with renal or hepatic disease because they accumulate.
Clinical Use
Vecuronium Bromide is usedmainly to produce skeletal muscle relaxation during surgeryand to assist in controlled respiration after general anesthesiahas been induced.
Side Effects
Common side effects of Vecuronium bromide include: muscle weakness, redness or inflammation at the injection site. Serious side effects include: measles, difficulty breathing, swelling of the face, lips, tongue, or throat; weak or shallow breathing, persistent muscle weakness, loss of movement in any part of the body, dizziness, and fever.
Veterinary Drugs and Treatments
Vecuronium is indicated as an adjunct to general anesthesia to produce muscle relaxation during surgical procedures or mechanical ventilation and to facilitate endotracheal intubation. It causes very minimal cardiac effects and generally does not cause the release of histamine.
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced muscle relaxant effect.
Anti-arrhythmics: procainamide enhances muscle
relaxant effect.
Antibacterials: effect enhanced by aminoglycosides,
clindamycin, polymyxins and piperacillin.
Antiepileptics: muscle relaxant effects antagonised by
carbamazepine; effects reduced by long-term use of
fosphenytoin and phenytoin but might be increased
by acute use.
Botulinum toxin: neuromuscular block enhanced
(risk of toxicity).
Metabolism
Vecuronium partly metabolised by the liver; the metabolites have some neuromuscular blocking activity. It is excreted mainly in bile as unchanged drug and metabolites; some is also excreted in the urine.
Properties of Vecuronium bromide
| Melting point: | 227-229°C |
| alpha | +30.5~+35.0゜(D/20℃)(c=0.5,dil.HCl) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Slightly soluble in water, freely soluble in methylene chloride, sparingly soluble in acetonitrile and in anhydrous ethanol. |
| form | neat |
| form | Solid |
| color | White |
| Merck | 14,9941 |
| BRN | 4837308 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 50700-72-6(CAS DataBase Reference) |
Safety information for Vecuronium bromide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Vecuronium bromide
| InChIKey | VEPSYABRBFXYIB-PWXDFCLTSA-M |
Vecuronium bromide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 VECURONIUM BROMIDE 99%View Details
VECURONIUM BROMIDE 99%View Details -
 Vecuronium Bromide 50700-72-6 98%View Details
Vecuronium Bromide 50700-72-6 98%View Details
50700-72-6 -
 Vecuronium Bromide CAS 50700-72-6View Details
Vecuronium Bromide CAS 50700-72-6View Details
50700-72-6 -
 Vecuronium bromide CAS 50700-72-6View Details
Vecuronium bromide CAS 50700-72-6View Details
50700-72-6 -
 Vecuronium Bromide CAS 50700-72-6View Details
Vecuronium Bromide CAS 50700-72-6View Details
50700-72-6 -
 Vecuronium bromide CAS 50700-72-6View Details
Vecuronium bromide CAS 50700-72-6View Details
50700-72-6 -
 Vecuronium Bromide 10 Mg InjectionView Details
Vecuronium Bromide 10 Mg InjectionView Details
50700-72-6 -
 Q VEC 4 Vecuronium Bromide Inj 4mg, 10mgView Details
Q VEC 4 Vecuronium Bromide Inj 4mg, 10mgView Details
50700-72-6
