Valperinol
- CAS NO.:64860-67-9
- Empirical Formula: C16H27NO4
- Molecular Weight: 297.39
- MDL number: MFCD00868249
- SAFETY DATA SHEET (SDS)
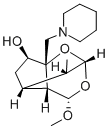
What is Valperinol?
Originator
Valperinol,ZYF Pharm Chemical
Manufacturing Process
Valperinol was prepared in 2 steps:
1). Preparation of 3-piperidinomethyl-4β-hydroxy-8-methoxy-10-methylene-
2,9-dioxatricyclo[4,3,1,03,7]decane: 500 ml of piperidine is added to 250 g of
sodium hydrogen carbonate and 190 g of 3-iodomethyl-4β-acetoxy-8-
methoxy-10-methylene-2,9-dioxatricyclo[4,3, 1,03,7]decane. The mixture is
heated to 150°C in an oil bath during 4 hours under thorough stirring and
reflux condenser cooling and then is cooled to room temperature. After adding
7.5 liter of ether, 1 liter of water is added for dissolving the mixture, then 200ml of a 40% sodium hydroxide solution is added and the mixture is shaken.
After separation of the etherical phase the aqueous phase is extracted 3 times
more with 500 ml of ether each. The united ether extracts are dried over
sodium sulfate and clarified with active carbon and filtered by suction, then
washed with ether. The filtrate is then evaporated in a rotation evaporator first
at 50°C under reduced pressure, which is produced by means of a water jet
pump and subsequently at 100°C under vacuum, which is produced by means
of an oil pump. Thereby 180 g of oily 3-piperidinomethyl-4β-hydroxy-8-
methoxy-10-methylene-2,9-dioxatricyclo[4,3,1,03,7]decane are obtained.
These are used without further purifying for the preparation of 3-
piperidinomethyl-4β-hydroxy-8-methoxy-10-methyl-2,9-
dioxatricyclo[4,3,1,03,7]decane.
2). Preparation of 3-piperidinomethyl-4β-hydroxy-8-methoxy-10-methyl-2,9-
dioxatricyclo[4 ,3,1,03,7]decane: A hydrogenation apparatus is flushed with
nitrogen for 10 minutes and then flushed with hydrogen for 10 minutes and
then is filled with nitrogen. 100 g of moist Raney nickel are washed into the
hydrogenation flask by means of methanol and are prehydrogenated under
low excess pressure and stirring for about 2 minutes at room temperature.
After introducing the solution of 180 g of the substance 3-piperidinomethyl-
4β-hydroxy-8-methoxy-10-methylene-2,9-dioxatricyclo[4,3,1,03,7]decane in
250 ml of methanol into the hydrogenation flask, there is further washed in a
mixing solution of sodium hydroxide, which is prepared by dissolving 20 g of
sodium hydroxide in a small amount of water, cooling this solution to room
temperature and diluting it with methanol to the five fold amount. The
mixture is hydrogenated under a low excess of pressure at room temperature
for about 30 minutes. After the hydrogen uptake has stopped, the mixture is
filtered over theorite through a suction filter which is then washed with
methanol (the catalyst must not become dry; danger of fire). 30 ml of acetic
acid are added to the filtrate, the solution is evaporated at 60oC., then cooled
to room temperature and the residue taken up in ether and worked into a
paste with 250 ml of silica gel (particle size 0.2-0.5 mm). After evaporation of
the solvent at 50°C, the residue is taken up in a n-hexane and subsequently
is evaporated at 60°C. The residue is filtered over a column of 500 g of silica
gel (particle size 0.2-0.5 mm) using first 1 liter of n-hexane and then n_x0002_hexane containing 1.5% diethylamine as an eluating solvent. After
evaporation of the filtrate at 60°C, 150 g of oily 3-piperidinomethyl-4β-
hydroxy-8-methoxy-10-methyl-2,9-dioxatricyclo[4,3,1,03,7]decane (valperinol)
are obtained. Empirical formula: C16H27NO4. Molecular weight: 297.399.
Therapeutic Function
Sedative, Antiepileptic, Antiparkinsonian
Safety information for Valperinol
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
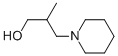
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4