Vagistat
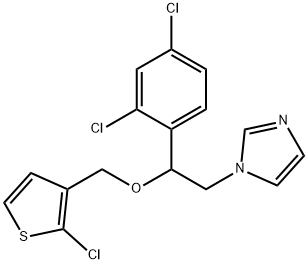
Synonym(s):1-[2-[(2-Chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole;Thioconazole;Tioconazole
- CAS NO.:65899-73-2
- Empirical Formula: C16H13Cl3N2OS
- Molecular Weight: 387.71
- MDL number: MFCD00057276
- EINECS: 265-973-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-28 18:20:31
What is Vagistat?
Absorption
Systemic absorption following a single intravaginal application of tioconazole in nonpregnant patients is negligible.
Toxicity
Symptoms of overdose include erythema, stinging, blistering, peeling, edema, pruritus, urticaria, burning, and general irritation of the skin, and cramps.
Description
Tioconazole is an antifungal agent, closely related to miconazole. Tioconazole is effective in the topical treatment of superficial fungal infections and appears to be more potent than miconazole against Candida and Trichophyton species.
Chemical properties
White powder EINECS: 265-973-8
Originator
Pfizer (United Kiugdom)
The Uses of Vagistat
antithrombotic
The Uses of Vagistat
Tioconazole is an antifungal that is more active than Fluconazole (F421000) or Voriconazole (V760000) against Candida glabrata mutant strains. Antifungal (topical).
The Uses of Vagistat
Potent inhibitor of cytochrome-P450
What are the applications of Application
Tioconazole is a cytochrome-P450 inhibitor
Indications
For the local treatment of vulvovaginal candidiasis (moniliasis).
Background
Tioconazole is an imidazole antifungal used to treat fungal and yeast infections. Topical formulations are used for ringworm, jock itch, athlete's foot, and tinea versicolor or "sun fungus". Tioconazole interacts with 14-alpha demethylase, a cytochrome P-450 enzyme that converts lanosterol to ergosterol, an essential component of the yeast membrane. In this way, tioconazole inhibits ergosterol synthesis, resulting in increased cellular permeability.
Definition
ChEBI: A member of the class of imidazoles that comprises 2-(2,4-dichlorophenyl)ethylimidazole carrying an additional (2-chloro-3-thienyl)methoxy substituent at position 2.
Indications
Topical treatment of mycoses of the skin induced or sustained by fungi such as dermatophytes and yeasts. In addition, tioconazole is indicated for therapy of vulvovaginal mycoses caused by Candida species and Candida (Torulopsis) glabrata.
Manufacturing Process
A solution of 1-(2,4-dichlorophenyl)-2-(1-imidazolyl)ethanol (1.5 g, 5.8 mmol) dissolved in dry tetrahydrofuran (10 ml) was added to a stirred suspension of sodium hydride (0.39 g, as 80% dispersion in oil, 16 mmol) in dry tetrahydrofuran (10 ml) and warmed to 70°C for 90 minutes. The mixture was cooled in ice and a solution of 2-chloro-3- chloromethylthiophene (8.8 mmol) in dry tetrahydrofuran was added. The mixture was heated at 70°C for 3 hours and allowed to stir at room temperature overnight. The solvent was removed under vacuum and the residue stirred with dry ether (200 ml). The ether solution was filtered through Celite and saturated with hydrogen chloride gas to precipitate an oil which was solidified by trituration with ether and ethyl acetate. The solid product was collected and recrystallized from a mixture of acetone and diisopropyl ether to give the product, melting point 168°C to 170°C.
brand name
TROSYL
Therapeutic Function
Antifungal
Antimicrobial activity
Tioconazole is a broad-spectrum imidazole antimycotic with activity against almost all species of pathogenic fungi.
Pharmacokinetics
Tioconazole is a broad-spectrum imidazole antifungal agent that inhibits the growth of human pathogenic yeasts. Tioconazole exhibits fungicidal activity in vitro against Candida albicans, other species of the genus Candida, and against Torulopsis glabrata. Tioconazole prevents the growth and function of some fungal organisms by interfering with the production of substances needed to preserve the cell membrane. This drug is effective only for infections caused by fungal organisms. It will not work for bacterial or viral infections.
Pharmacokinetics
Tioconazole is normally not absorbed after topical application to the skin [173]. On vaginal application of an ovulum containing 300 mg tioconazole, a serum concentration of 21 μg/mL was observed after 8 h.
Clinical Use
1-[2-[(2-chloro-3-thienyl)methoxy]2-(2,4-dichlorophenyl)-ethyl]-1H-imidazole (Vagistat) is used for the treatment ofvulvovaginal candidiasis. A vaginal ointment containing6.5% of the free base is available. Tioconazole is more effectiveagainst Torulopsis glabrata than are other azoles.
Side Effects
Local irritations such as itching, burning sensations, erythema, and dermatitis, and allergic reactions may occur in rare cases and are mainly due to the galenic formulation.
Metabolism
Orally administered tioconazole is extensively metabolized. The major metabolites are glucuronide conjugates.
Properties of Vagistat
| Melting point: | 79-81oC |
| Boiling point: | 534.5±50.0 °C(Predicted) |
| Density | 1.3115 (rough estimate) |
| RTECS | NI4480000 |
| refractive index | 1.6000 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Very slightly soluble in water, very soluble in methylene chloride, freely soluble in alcohol. |
| form | powder |
| pka | 6.66±0.12(Predicted) |
| color | white to off-white |
| CAS DataBase Reference | 65899-73-2(CAS DataBase Reference) |
Safety information for Vagistat
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Vagistat
Vagistat manufacturer
Solara Active Pharma Sciences Ltd
Optimus Pharma Pvt Ltd (Sekhmet Pharmaventures))
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![Tioconazole Related Compound A (25 mg) (1-[2,4-Dichloro-beta-[(3-thenyl)-oxy]phenethyl]imidazole hydrochloride)](https://img.chemicalbook.in/CAS/20180808/GIF/61709-33-9.gif)



You may like
-
 65899-73-2 TIOCONAZOLE 98%View Details
65899-73-2 TIOCONAZOLE 98%View Details
65899-73-2 -
 Tioconazole 99%View Details
Tioconazole 99%View Details -
 Tioconazole 65899-73-2 98%View Details
Tioconazole 65899-73-2 98%View Details
65899-73-2 -
 Tioconazole 98%View Details
Tioconazole 98%View Details
65899-73-2 -
 65899-73-2 98%View Details
65899-73-2 98%View Details
65899-73-2 -
 Tioconazole 98%View Details
Tioconazole 98%View Details
65899-73-2 -
 Tioconazole 98% CAS 65899-73-2View Details
Tioconazole 98% CAS 65899-73-2View Details
65899-73-2 -
 Tioconazole CAS 65899-73-2View Details
Tioconazole CAS 65899-73-2View Details
65899-73-2