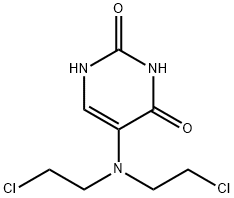
URACIL MUSTARD
- CAS NO.:66-75-1
- Empirical Formula: C8H11Cl2N3O2
- Molecular Weight: 252.1
- MDL number: MFCD00085645
- EINECS: 200-631-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:44:01
What is URACIL MUSTARD ?
Description
Uracil mustard appears as creamy/off-white, odourless, crystalline powder. It is used as an anti-cancer medicine. Uracil mustard is a chemotherapy drug that belongs to the class of alkylating agents. It is used for its anti-neoplastic properties. It works by damaging deoxyribonucleic acid (DNA), primarily in cancer cells that preferentially take up the uracil due to their need to make nucleic acids during their rapid cycles of cell division. At high concentrations of the drug, cellular RNA and protein synthesis are also suppressed. The DNA damage leads to apoptosis of the affected cells. Chemically it is a derivative of nitrogen mustard and uracil. Uracil mustard is a non-combustible substance itself; it does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidisers and may ignite combustibles – wood, paper, oil, clothing, etc. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Originator
Uracil Mustard,Upjohn,US,1962
The Uses of URACIL MUSTARD
Antineoplastic.
Background
Nitrogen mustard derivative of uracil. It is a alkylating antineoplastic agent that is used in lymphatic malignancies, and causes mainly gastrointestinal and bone marrow damage.
Indications
Used for its antineoplastic properties.
Definition
ChEBI: 5-[bis(2-chloroethyl)amino]uracil is a nitrogen mustard and an aminouracil.
Manufacturing Process
Preparation of 5-[bis(2-Hydroxyethyl)Amino] Uracil: 20 grams (0.157 mol) of
5-aminouracil was mixed with 350 ml of water, 23 ml of glacial acetic acid,
and 160 ml of ethylene oxide in a one-liter flask immersed in an ice bath. The
reaction mixture was stirred and allowed to come to room temperature slowly
(as the ice melted), and stirring was continued for two days. A clear solution
resulted to which was added 250 ml of water and 60 grams of Dowex-50 in
the acid form. The mixture was stirred for 15 minutes, and the resin was
collected on a filter. It was washed with water and the crude 5-[bis(2-
hydroxyethyl)amino] uracil was eluted with a 10% aqueous solution of
ammonium hydroxide. This eluate was evaporated to dryness, and the solid
that remained was heated with 350 milliliters of isopropyl alcohol.Undissolved substances were removed by filtration and the filtrate was
concentrated on a steam bath to a volume of about 125 ml and cooled to
effect crystallization. After 20 hours at room temperature the crystals that had
formed were recovered, washed with isopropyl alcohol, and dried, yielding
15.61 grams (46.2%) of crystalline 5-[bis(2-hydroxyethyl)amino] uracil having
a MP of 157° to 163°C. An analytical sample, obtained by several
recrystallizations from isopropyl alcohol, melted at 166° to 168°C.
Preparation of 5-[bis(2-Chloroethyl)Amino] Uracil: 13 ml of thionyl chloride
was added to 52 ml of diethylene glycol dimethyl ether accompanied by
stirring. Heat was generated, and sulfur dioxide and hydrogen chloride were
liberated. The mixture was cooled and 5.58 grams of 5-[bis(2-
hydroxyethyl)amino] uracil was added, followed by 8 ml of thionyl chloride, No
evidence of reaction was noted, and the reaction mixture was heated to about
40°C, gas then being evolved. After one hour at 40°C, 5 ml of thionyl chloride
was added, and after 30 minutes, another 3 ml was added. The mixture was
then heated to 55°C, whereupon it darkened and all of the solid dissolved.
After cooling and storage at room temperature for 20 hours, three volumes of
benzene was added and a dark solid precipitated. After one hour, the dark
solid was collected on a filter, washed with benzene, and dissolved in a
minimum of boiling methanol. Crystals formed upon cooling; and after 18
hours in the refrigerator, they were recovered on a filter, washed with cold
methanol, and dried under reduced pressure, yielding 2.96 grams of 5-[bis(2-
chloroethyl)amino] uracil. The product was recrystallized by dissolving in a
minimum of hot methanol and adding water until the solution became cloudy;
2.25 grams of 5-[bis(2-chloroethyl)amino] uracil was recovered after cooling
the mixture to 4°C for 16 hours (MP 200° to 205°C). A small sample was
recrystallized again, and it melted at 198° to 204°C.
Therapeutic Function
Cancer chemotherapy
General Description
Creamy white crystals or off-white powder. Used as an anti-cancer medicine.
Air & Water Reactions
Slightly soluble in water [Merck].
Reactivity Profile
URACIL MUSTARD (500 MG) (FOR U.S. SALE ONLY) reacts as a base.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Pharmacokinetics
Uracil Mustard selectively inhibits the synthesis of deoxyribonucleic acid (DNA). The guanine and cytosine content correlates with the degree of Uracil Mustard-induced cross-linking. At high concentrations of the drug, cellular RNA and protein synthesis are also suppressed.
Safety Profile
Suspected carcinogen withexperimental carcinogenic and neoplastigenic data. Adeadly poison by ingestion and intraperitoneal routes.Mutation data reported. When heated to decomposition itemits very toxic fumes of Cl?? and NOx.
Carcinogenicity
Uracil mustard was reportedly carcinogenic in both mice and rats following multiple intraperitoneal injections. It produced a dose-related increase in lung tumor incidence in mice and tumors in a variety of other organs in both mice and rats. The IARC reviewed the preceding data and deemed it “sufficient evidence of carcinogenicity in animals.” Based on this information, its mutagenic potential, analogy to other nitrogen mustards, and a lack of carcinogenicity data in humans, the IARC classified uracil mustard in Group 2B (possibly carcinogenic to humans). Uracil mustard compared to nitrogen mustard itself in the same assay (lung tumor assay in strain A mice, intraperitoneal dosing) was found to be more potent as a tumorigen than nitrogen mustard. An in vitro assay to predict carcinogenicity gave a positive response predicting that uracil mustard would be a carcinogen in rodent test models. The assay used focus formation in a stable bovine papillomavirus type 1 DNA carrying a mouse fibroblast cell line that does not require transfection, infection with virus,isolation of primary cells from animals, or addition of a microsomal fraction.
Metabolism
Not Available
Properties of URACIL MUSTARD
| Melting point: | 206°C (rough estimate) |
| Density | 1.6350 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| solubility | DMSO (Sparingly), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 9.42±0.10(Predicted) |
| color | Crystals from MeOH (aq) |
| IARC | 2B (Vol. 9, Sup 7) 1987 |
| EPA Substance Registry System | Uracil mustard (66-75-1) |
Safety information for URACIL MUSTARD
Computed Descriptors for URACIL MUSTARD
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
![2-[bis(2-chloroethyl)amino]acetaldehyde](https://img.chemicalbook.in/CAS/GIF/102585-22-8.gif)
![2,6-DIHYDROXY-4-METHYL-5-[BIS(2-CHLOROETHYL)AMINO]PYRIMIDINE](https://img.chemicalbook.in/CAS/GIF/520-09-2.gif)

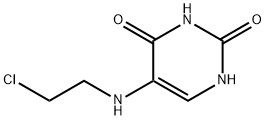
You may like
-
 5-(BIS-(2-CHLOROETHYL)-AMINO)-URACIL CAS 66-75-1View Details
5-(BIS-(2-CHLOROETHYL)-AMINO)-URACIL CAS 66-75-1View Details
66-75-1 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4