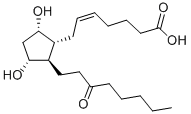
UNOPROSTONE
- CAS NO.:120373-36-6
- Empirical Formula: C22H38O5
- Molecular Weight: 382.53
- MDL number: MFCD00865635
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
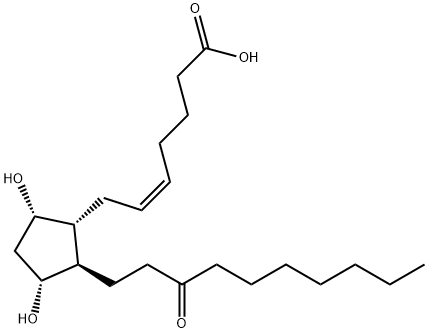
What is UNOPROSTONE?
Absorption
After application to the eye, unoprostone isopropyl is absorbed through the cornea and conjunctival epithelium where it is hydrolyzed by esterases to unoprostone free acid. mean peak unoprostone free acid plasma concentration was <1.5 ng/mL and dro pped below the lower limit of quantitation (<0.250 ng/mL) 1 hour following instillation, indicating low systemic absorption and rapid plasma excretion.
Toxicity
LD50 in rats: 1g/Kg (subcutaneous), 93500 μg/Kg (IV).
The Uses of UNOPROSTONE
Unoprostane is a protectant against retinal degeneration in S334ter rhodopsin mutant rats. Neuroprotective agent.
Indications
For the lowering of intraocular pressure in patients with open-angle glaucoma or ocular hypertension who are intolerant of other intraocular pressure lowering medications or insufficiently responsive (failed to achieve target IOP determined after multiple measurements over time) to another intraocular pressure lowering medication.
Background
Unoprostone isopropyl is a prostaglandin analogue. Ophthalmic Solution 0.15% is a synthetic docosanoid. Unoprostone isopropyl has the chemical name isopropyl (+)-(Z)-7-[(1R,2R,3R,5S)-3,5 dihydroxy-2-(3-oxodecyl)cyclopentyl]-5-heptenoate. The main indication of Unoprostane is treatment of glucoma.
What are the applications of Application
Unoprostone is a PGF2α analog
Definition
ChEBI: Unoprostone is an oxo monocarboxylic acid, a prostaglandins Falpha and a ketone. It has a role as an antiglaucoma drug and an antihypertensive agent.
General Description
Unoprostone (Rescula) is supplied as a 0.15% sterileophthalmic solution. Unoprostone is somewhat unusual inthat it is a docosanoid (22-carbon atom) PGF2α analog marketedas the isopropyl ester. The natural 15-position alcoholis oxidized to the ketone as would be expected to occur invivo. Cautions and side effects are similar to those givenpreviously.
Pharmacokinetics
Unoprostone will begin to reduce IOP 30 minutes after ocular instillation.
Metabolism
After ocular application, unoprostone isopropyl is hydrolyzed by esterases in the cornea to its biological active metabolite, unoprostone free acid. Unoprostone free acid is then metabolized to several inactive metabolites with lower molecular weight and increased polarity via ε- or β-oxidation. No secondary conjugation is found and no significant effect on hepatic microsomal enzyme activity has been observed.
Properties of UNOPROSTONE
| Boiling point: | 562.9±40.0 °C(Predicted) |
| Density | 1.069±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMF: 25 mg/ml; DMSO: 17 mg/ml; Ethanol: 12 mg/ml; PBS (7.2): 90 μg/ml |
| pka | 4.76±0.10(Predicted) |
Safety information for UNOPROSTONE
Computed Descriptors for UNOPROSTONE
New Products
(R)-3-Aminobutanenitrile Hydrochloride 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION AMOXICILLIN (AMOXYCILLIN) TRIHYDRATE ACICLOVIR AMLODIPINE SODIUM METHYL PARABEN Methylcobalamin (vitamin B12) SODIUM VALPROATE 5-Fluoro-2-iodo-3-methylaniline Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 3-Bromo-2-hydroxybenzeneacetic acid 5-Iodo-3-methyl-2-nitropyridine 1-(3-Chloro-2-hydroxyphenyl)-2-propanoneRelated products of tetrahydrofuran


You may like
-
![446292-08-6 (S)-2-{2-Oxo-3-[4-(3-oxo-morpholin-4-yl)-phenyl]-oxazolidin-5-ylmethyl}-isoindole-1,3-dione 99](https://img.chemicalbook.in//Content/image/CP5.jpg) 446292-08-6 (S)-2-{2-Oxo-3-[4-(3-oxo-morpholin-4-yl)-phenyl]-oxazolidin-5-ylmethyl}-isoindole-1,3-dione 99View Details
446292-08-6 (S)-2-{2-Oxo-3-[4-(3-oxo-morpholin-4-yl)-phenyl]-oxazolidin-5-ylmethyl}-isoindole-1,3-dione 99View Details
446292-08-6 -
 494-19-9 >99View Details
494-19-9 >99View Details
494-19-9 -
 Iminostilbene 256-96-2 98View Details
Iminostilbene 256-96-2 98View Details
256-96-2 -
 147403-52-9 > 99View Details
147403-52-9 > 99View Details
147403-52-9 -
 5,6-Dimethoxy-2-(4-piperidinylmethyl)-1-indanone hydrochloride 120013-39-0 > 99.0View Details
5,6-Dimethoxy-2-(4-piperidinylmethyl)-1-indanone hydrochloride 120013-39-0 > 99.0View Details
120013-39-0 -
![718635-93-9 3-(dimethylamino)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-one >99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 718635-93-9 3-(dimethylamino)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-one >99%View Details
718635-93-9 3-(dimethylamino)-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-one >99%View Details
718635-93-9 -
![120014-30-4 5,6-Dimethoxy-2-[(4-piperidyl) methyl]indan-1-one > 99.0](https://img.chemicalbook.in//Content/image/CP5.jpg) 120014-30-4 5,6-Dimethoxy-2-[(4-piperidyl) methyl]indan-1-one > 99.0View Details
120014-30-4 5,6-Dimethoxy-2-[(4-piperidyl) methyl]indan-1-one > 99.0View Details
120014-30-4 -
 147403-65-4 > 99View Details
147403-65-4 > 99View Details
147403-65-4