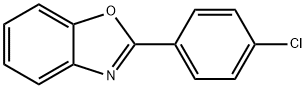
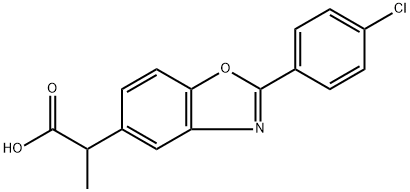
Uniprofen
- CAS NO.:51234-28-7
- Empirical Formula: C16H12ClNO3
- Molecular Weight: 301.73
- EINECS: 257-069-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is Uniprofen?
Originator
Opren,Dista Lilly,UK,1980
The Uses of Uniprofen
A non-steroidal anti-inflammatory ophthalmic agent.
Background
The use of benoxaprofen, formerly marketed as Oraflex tablets, was associated with fatal cholestatic jaundice among other serious adverse reactions. The holder of the approved application voluntarily withdrew Oraflex tablets from the market on August 5, 1982.
Definition
ChEBI: A monocarboxylic acid that is propionic acid substituted at position 2 by a 2-(4-chlorophenyl)-1,3-benzoxazol-5-yl group. It was used as a non-steroidal anti-inflammatory drug until 1982 when it was withdrawn from the market due to adverse side-effects inc uding liver necrosis, photosensitivity, and carcinogenicity in animals.
Manufacturing Process
The 6-benzoxazolyl analog of the 5-benzoxazolyl product is prepared as
follows:
(a) Ethyl 2-(2-p-chlorophenyl-6-benzoxazolyl)propionate: A solution of ethyl
2-(3-hydroxy-4-aminophenyl)propionate (2.5 g) in pyridine (15 ml) was
treated with p-chlorobenzoyl chloride (1.65 ml) at 5°C. After stirring for 2
hours at room temperature the solution was evaporated to dryness.
The residue was heated at 220°C until no more water was evolved, then was
allowed to cool. This yielded ethyl 2-(2-p-Chlorophenyl-6-
benzoxazolyl)propionate.
(b) 2-(2-p-Chlorophenyl-6-benzoxazolyl)propionic acid: A solution of ethyl 2-
(2-chlorophenyl-6-benzoxazolyl)propionate (4 g) in aqueous sodium hydroxide
(30 ml) was heated on a steam bath for one-half hour. On cooling the black
solution was washed with chloroform. On acidification of the black solution
with hydrochloric acid the mixture was extracted with chloroform. This
solution on evaporation yielded 2-(2-p-chlorophenyl-6-benzoxazolyl)propionic
acid, MP 196°C.
brand name
Oraflex (Lilly);90459 compound;Benoxapran;Bexopron;Compound 90459;Lilly 3794;Lilly 90459;Lrcl 3794;Oprenal.
Therapeutic Function
Antiinflammatory, Analgesic
World Health Organization (WHO)
Benoxaprofen, a nonsteroidal antiinflammatory agent, was introduced in 1980 for the treatment of rheumatic disorders. Following reports of serious adverse effects, some of which were fatal, it was withdrawn in several countries prior to worldwide withdrawal by the manufacturer in 1982.
Safety Profile
Poison by ingestion, subcutaneous, and intraperitoneal routes. Moderately toxic to humans by ingestion.Human systemic effects by ingestion: jaundice and gynecomastia (excessive development of the male mammary glands), changes in kidney tubules, decreased
Metabolism
Not Available
Properties of Uniprofen
| Melting point: | 189.5°C |
| Boiling point: | 446.2±30.0 °C(Predicted) |
| Density | 1.2411 (rough estimate) |
| refractive index | 1.6470 (estimate) |
| storage temp. | Refrigerator |
| solubility | DMSO: slightly; Methanol: slightly, sonicated |
| form | A solid |
| pka | 4.36±0.30(Predicted) |
| color | Cream solid from EtOH |
Safety information for Uniprofen
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
Computed Descriptors for Uniprofen
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8