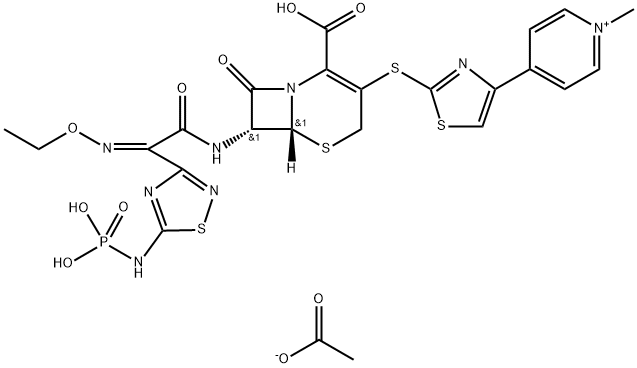
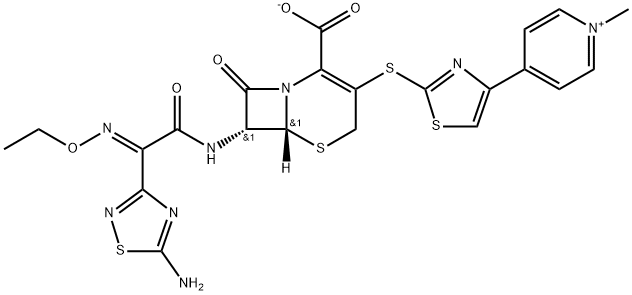
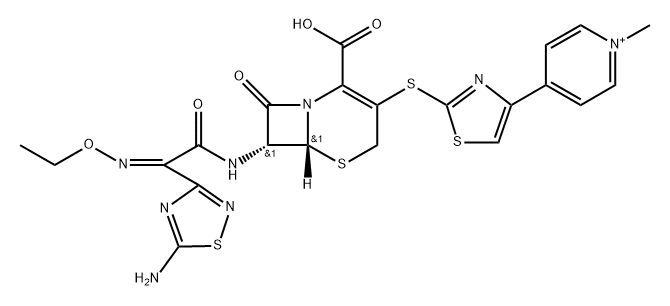
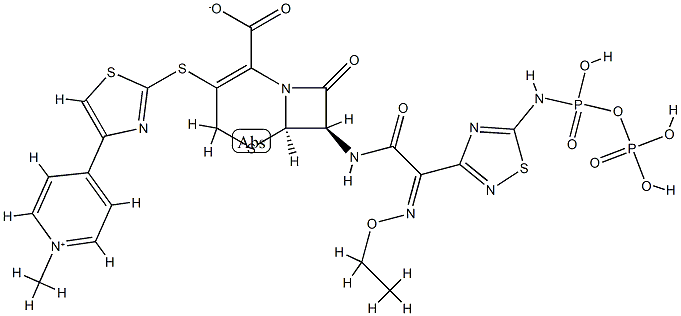
TAK 599
- CAS NO.:400827-46-5
- Empirical Formula: C24H25N8O10PS4
- Molecular Weight: 744.72
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-24 14:33:46
What is TAK 599?
Description
Ceftaroline fosamil, also referred to as TAK-599, is a cephalosporin antibacterial agent that was approved in the United States in October 2010 for the IV treatment of acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP). Ceftaroline fosamil is the water-soluble, N-phosphono prodrug of ceftaroline (T-91825), a broad-spectrum, bactericidal agent with potent activity against methicillin-resistant Staphylococcus aureus (MRSA) strains, multidrug resistant S. pneumonia, and common gram-negative organisms. Ceftaroline binds to PBP2a as well as other PBPs with high affinity and, as a result, retains potent activity. Ceftaroline exhibits activity against most gram-positive pathogens, including β-lactam-susceptible and -resistant S. aureus, vancomycin-resistant S. aureus, and resistant and susceptible forms of S. pneumoniae but has weak activity against Enterococcus sp. The gram-negative antibacterial activity of ceftaroline is limited mainly to respiratory pathogens such as Moraxella catarrhalis and Haemophilus influenzae.
Originator
Takeda (Japan)
Definition
ChEBI: An acetate salt obtained by reaction of ceftaroline fosamil with one equivalent of acetic acid. A prodrug for ceftaroline, used for the treatment of adults with acute bacterial skin and skin structure infections.
brand name
Teflaro
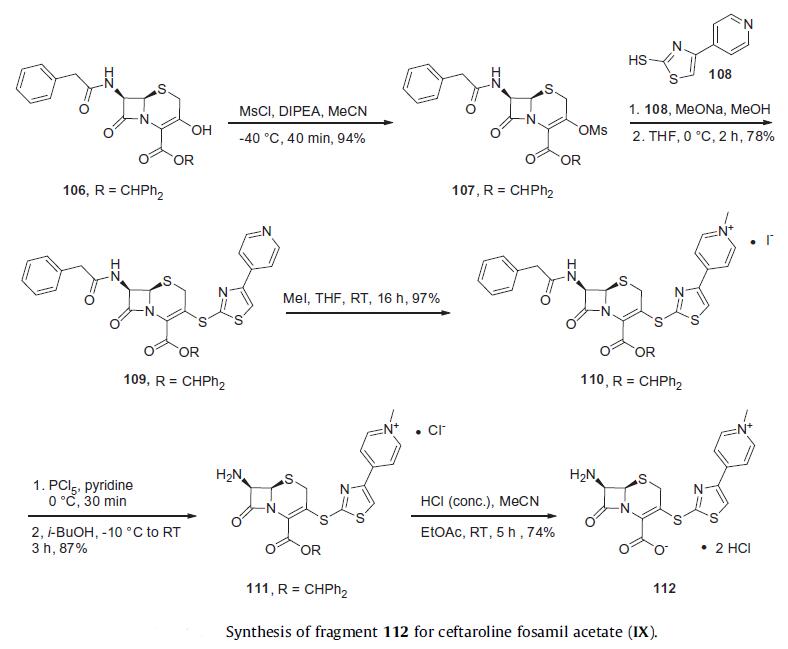
Synthesis
Reports from Takeda describe a process preparation of ceftaroline
fosamil in 100 g scale which relies upon the assembly and union
of fragments 112 and 114. The
synthesis of fragment 112 began from commercially available
benzhydryl 7b-[(phenylacetyl)amino]-3-hydroxy-3-cephem-4-
carboxylate (106). The hydroxyl group within cephem 106 was reacted
with methanesulfonyl chloride to produce mesylate 107 in
94% yield. The condensation of mesylate 107 with 4-(pyridin-
4-yl)thiazole-2-thiol 108 under the base condition of sodium
methoxide gave compound 109 in 78% yield. Pyridinium salt 110 arose in quantitative yield upon subjection of 109 to iodomethane.
Sequential deprotections of the amino group with phosphorous
pentachloride and ester group with concentrated HCl afforded
the dihydrochloride salt 112 in good yield.

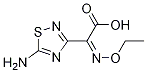
Acyl halide fragment 114 was prepared from commercially
available (Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-ethoxyiminoacetic
acid (113) in 60% yield by dichlorophosphorylation of the amino
group and concomitant acid chloride formation. Acid chloride 114
was then reacted with dihydrochloride salt 112 in the presence of
sodium acetate to give N-phosphono cephem 115 in 77% yield. The
crystallization of 115 in an aqueous acetic acid solution gave rise to
the stable acetic acid solvate ceftaroline fosamil acetate (IX).
Drug interactions
Potentially hazardous interactions with other drugs
Anticoagulants: effects of coumarins may be
enhanced.
Metabolism
Ceftaroline fosamil (prodrug) is converted into the active
ceftaroline in plasma by phosphatase enzymes. Hydrolysis
of the beta-lactam ring of ceftaroline occurs to form
the microbiologically inactive, open-ring metabolite,
ceftaroline M-1.
Ceftaroline is mainly eliminated by the kidneys. Renal
clearance is approximately equal, or slightly lower than
the glomerular filtration rate in the kidney, and in vitro
transporter studies indicate that active secretion does not
contribute to the renal elimination of ceftaroline.
Properties of TAK 599
| storage temp. | Store at -20°C |
| solubility | DMSO : 30 mg/mL (40.28 mM; Need ultrasonic and warming) |
| form | Powder |
| color | White to light yellow |
Safety information for TAK 599
Computed Descriptors for TAK 599
TAK 599 manufacturer
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 400827-46-5 Ceftaroline fosamil 98%View Details
400827-46-5 Ceftaroline fosamil 98%View Details
400827-46-5 -
 Ceftaroline fosamil acetate hydrate CAS 400827-46-5View Details
Ceftaroline fosamil acetate hydrate CAS 400827-46-5View Details
400827-46-5 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
