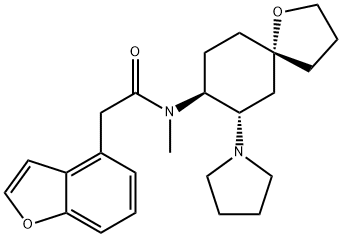
U-69593
Synonym(s):(+)-(5α,7α,8β)-N-Methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide;U69593
- CAS NO.:96744-75-1
- Empirical Formula: C22H32N2O2
- Molecular Weight: 356.5
- MDL number: MFCD05664586
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-26 14:13:49
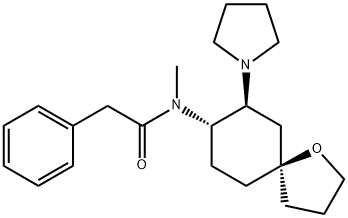
What is U-69593?
The Uses of U-69593
U-69593 is a selective κ-opioid receptor agonist. A safe opioid analgesic.
What are the applications of Application
U-69593 is a selective κ-opioid receptor agonist
Definition
ChEBI: U69593 is a monocarboxylic acid amide obtained by formal condensation between the carboxy group of phenylacetic acid and the secodary amino group of (5R,7S,8S)-N-methyl-7-(pyrrolidin-1-yl)-1-oxaspiro[4.5]decan-8-amine. It has a role as a kappa-opioid receptor agonist, an anti-inflammatory agent and a diuretic. It is an oxaspiro compound, a N-alkylpyrrolidine, an organic heterobicyclic compound and a monocarboxylic acid amide.
Biological Functions
U-69593 is a single enantiomer with very high selectivity for the kappa receptor in vitro (mu/kappa ratio = 484). It was radiolabelled by a catalytic exchange of tritium for the two aromatic chlorine substituents of the precursor. This ligand is commercially available and has been widely used as a radiolabel for the kappa receptor.
in spinal cordslice preparations from the 9-16-day-old rat,U-69593 produced a na-loxone-reversible depression of spontaneous and electrically evoked activityin dorsal horn neurones.
Biochem/physiol Actions
U-69593 is a selective κ opioid receptor agonist. U-69593 is known to inhibit cocaine sensitization in meso-limbic dopamine neurons by normalizing basal overflow of dopamine.
Structure and conformation
An X-ray analysis of the crystal structures of U-50488 and U-69593 have been determined. The cyclohexane ring adopts a chair conformation with both nitrogen substituents attached equatorially The amide bond is in a trans conformation and the absolute stereochemistry of the kappa opioid active enantiomer of U-50488 is [S,S]. There appears to be some confusion concerning the ab-solute stereochemistry of U-69593.The structure in the publication describ-ing the X-ray crystallography is drawn as 5R,7S,8S,but the title of thispaper,which gives the full chemical name of U-69593, indicates 5S,7S,8S.
Properties of U-69593
| Melting point: | 120-123℃ |
| Boiling point: | 518.7±50.0 °C(Predicted) |
| Density | 1.13 |
| RTECS | CY1490060 |
| storage temp. | 2-8°C |
| solubility | H2O: 14 mg/mL |
| form | solid |
| pka | 9.15±0.20(Predicted) |
| color | white |
| optical activity | [α]/D +7.8°, c = 0.825 in methanol(lit.) |
Safety information for U-69593
Computed Descriptors for U-69593
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![U-69,593, [PHENYL-3,4-3H]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB4430816.gif)
You may like
-
 U-69593 >95% CAS 96744-75-1View Details
U-69593 >95% CAS 96744-75-1View Details
96744-75-1 -
 U-69593 CAS 96744-75-1View Details
U-69593 CAS 96744-75-1View Details
96744-75-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1