Trioctylphosphine oxide
Synonym(s):(Oct)3PO;Thorium ionophore I;TOPO;Trioctylphosphinoxide
- CAS NO.:78-50-2
- Empirical Formula: C24H51OP
- Molecular Weight: 386.63
- MDL number: MFCD00002083
- EINECS: 201-121-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-09 18:15:22
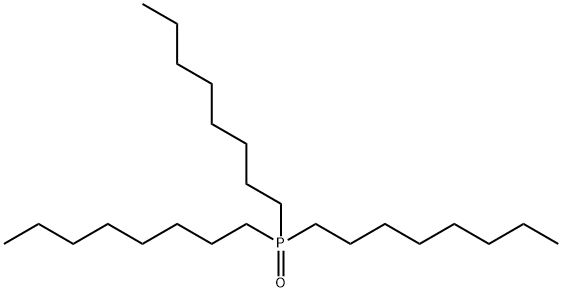
What is Trioctylphosphine oxide?
Chemical properties
white crystalline powder
The Uses of Trioctylphosphine oxide
Trioctylphosphine oxide (TOPO) is most commonly used as the solvent due to its high boiling point and chemical stability, and its ability to prevent particle aggregation via coordination to the NP surface. trioctylphosphine oxide is a frequently used surfactant, starts to decompose at around 425°C.
The industrial applications of trioctylphosphine oxide, make use of its complexing powers with metals and with hydrogen donor organic compounds. Commercial uses as a solvent extraction reagent are in the recovery of uranium from wet process phosphoric acid and in the recovery of byproduct acetic acid and furfural generated during sulphite wood pulping.
TOPO is a widely used chemical compound in nanocrystal synthesis, for the removal of heavy metals, and the removal of toxins in waste water. TOPO is often used as a ligand stabilizer for colloids in traditional thermal decomposition synthetic techniques.
trioctylphosphine oxide became a nearly irreplaceable solvent (mp 51-52°C) for high-temperature preparation of various type of nanocomposites. TOPO has a very high boiling point (411°C) which is an important prerequisite for the homogeneous nucleation and good crystallinity of the nanoparticles.
The Uses of Trioctylphosphine oxide
Tri-n-octylphosphine oxide is a solvent used in the extraction of metals, especially uranium, zirconium and hafnium. It is also used as an auxiliary reagent in the solvent extraction of metals. It is also used to extract hydrogen bonding organic compounds. Further, it is useful as a capping ligand for the production of quantum dots, such as those consisting of cadmium selenide.
The Uses of Trioctylphosphine oxide
Catalyst in:
- Preparation of tetradentate planar-chiral hydroxy-substituted ferrocenecarboxaldimine Schiff base ligands
- Reactions of allyl esters with hydrosilanes
- Asymmetric cyanosilylation of aldehydes
- Preparation of chlorothiol formates from thiols and phosgene
What are the applications of Application
Trioctylphosphine oxide is a capping ligand for quantum dots to stabilize nanoparticles in organic solutions
General Description
Visit our Sensor Applications portal to learn more.
Flammability and Explosibility
Non flammable
Purification Methods
Mason, McCarty and Peppard [J Inorg Nuclear Chem 24 967 1962] stirred a 0.1M solution in *benzene with an equal volume of 6M HCl at 40o in a sealed flask for 48hours, then washed the *benzene solution successively with water (twice), 5% aqueous Na2CO3 (three times) and water (six times). The *benzene and water were then evaporated under reduced pressure at room temperature. Zingaro and White [J Inorg Nucl Chem 12 315 1960] treated a pet ether solution with aqueous KMnO4 (to oxidise any phosphinous acids to phosphinic acids), then with sodium oxalate, H2SO4 and HCl (to remove any manganese compounds). The pet ether solution was slurried with activated alumina (to remove phosphinic acids), filtered, evaporated and the residue was recrystallised from pet ether or cyclohexane at -20o. It can also be recrystallised from EtOH. [Beilstein 4 IV 3466.]
Properties of Trioctylphosphine oxide
| Melting point: | 50-52 °C(lit.) |
| Boiling point: | 201-202 °C2 mm Hg(lit.) |
| Density | 0,88 g/cm3 |
| vapor pressure | 0.004Pa at 50℃ |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | <1g/l |
| form | Crystalline Powder, Crystals or Flakes |
| color | White to slightly yellow |
| Water Solubility | Insoluble |
| BRN | 1796648 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 78-50-2(CAS DataBase Reference) |
| EPA Substance Registry System | Phosphine oxide, trioctyl- (78-50-2) |
Safety information for Trioctylphosphine oxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for Trioctylphosphine oxide
Trioctylphosphine oxide manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Trioctylphosphine oxide, GR 99%+ CAS 78-50-2View Details
Trioctylphosphine oxide, GR 99%+ CAS 78-50-2View Details
78-50-2 -
 Tri-n-octylphosphine Oxide CAS 78-50-2View Details
Tri-n-octylphosphine Oxide CAS 78-50-2View Details
78-50-2 -
 Trioctylphosphine oxide, technical grade CAS 78-50-2View Details
Trioctylphosphine oxide, technical grade CAS 78-50-2View Details
78-50-2 -
 TRI-n-OCTYL PHOSPHINE OXIDE AR CAS 78-50-2View Details
TRI-n-OCTYL PHOSPHINE OXIDE AR CAS 78-50-2View Details
78-50-2 -
 Trioctylphosphine oxide CAS 78-50-2View Details
Trioctylphosphine oxide CAS 78-50-2View Details
78-50-2 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
