TRIOCTANOIN
Synonym(s):Glycerol tricaprylate;Tricaprylin;Trioctanoin;Glyceryl trioctanoate;Glycerol trioctanoate
- CAS NO.:538-23-8
- Empirical Formula: C27H50O6
- Molecular Weight: 470.68
- MDL number: MFCD00036236
- EINECS: 208-686-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
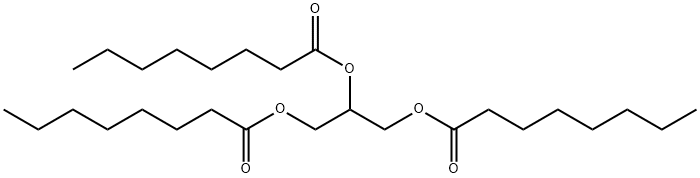
What is TRIOCTANOIN?
Description
1,2,3-Trioctanoyl glycerol is a triacylglycerol that contains octanoic acid at the sn-1, sn-2, and sn-3 positions. Dietary administration of 1,2,3-trioctanoyl glycerol (260 g/kg diet) increases hepatic and adipose tissue glucose-6-phosphate dehydrogenase (G6PDH), citrate cleavage enzyme (CCE), and malic enzyme activities in rats. It induces nuclear edema and cytolysis in tumor cells, but not normal hepatic cells, in a murine hepatic carcinoma model.
Chemical properties
clear colorless to yellow viscous liquid
Chemical properties
Tricaprylin occurs as a clear, colorless to pale-yellow liquid. It forms crystals from acetone/ethanol (95%). Tricaprylin is odorless.
The Uses of TRIOCTANOIN
Glytex(R) 273 is a low viscosity polyol ester suggested for use as a synthetic fiber lubricant. It offers a balance of moderate volatility and low varnishing behavior. For low to moderate denier polyester and nylon filament and staple yarn applications. Product Data Sheet
The Uses of TRIOCTANOIN
glyceryl trioctanoate is an emollient with skin-softening abilities.
The Uses of TRIOCTANOIN
tricaprylin is a skin-conditioning agent.
What are the applications of Application
Glyceryl trioctanoate is a triglyceride of octanoic acid esters
Definition
ChEBI: A triglyceride obtained by acylation of the three hydroxy groups of glycerol by octanoic acid. Used as an alternative energy source to glucose for patients with mild to moderate Alzheimer's disease.
Production Methods
Tricaprylin is a triglyceride manufactured by esterification of caprylic acid and glycerin.
General Description
Odorless viscous clear colorless to amber-brown liquid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
TRIOCTANOIN is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. TRIOCTANOIN is incompatible with strong oxidizers. .
Fire Hazard
TRIOCTANOIN is combustible.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Tricaprylin is used in pharmaceutical preparations as a neutral
carrier, absorption promoter, and solubilizer for active drugs. It has
been used as an oily phase to prepare water-in-oil-in-water multiple
emulsions for incorporating water-soluble drugs such as cefadroxil, cephradine, 4-aminoantipyrine, and antipyrine, and also for
obtaining stable microcapsules.
Tricaprylin acts as a vehicle for topical creams and lotions, and
cosmetic preparations. It is used as a penetration-enhancing lipid
base with excellent emollient and skin-smoothing properties.
Owing to its non-greasy components and low viscosity, it has very
good spreadability. In spite of being skin-permeable, tricaprylin
does not obstruct natural skin respiration, and hence it is used in
baby oils, massage oils, and face masks. It is an excellent dispersant,
and acts as a solubilizer, wetting agent and binder in color
cosmetics. Being readily miscible with natural oils and surfactants,
tricaprylin is used as the fat component in two-phase foam baths. It
is used in sunscreen creams and oils because of its compatibility with
organic and inorganic filter agents. It is also used as a fixative for
perfumes/fragrances.
Biochem/physiol Actions
Glyceryl trioctanoate might serve as a skin softening agent. It possesses caprylic acid as the aliphatic chain.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by intravenous route. Mildly toxic by ingestion. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.
Safety
Tricaprylin is used in pharmaceutical and cosmetic formulations.
The Cosmetic Ingredient Review (CIR) Expert Panel found that
dermal application of tricaprylin has not been associated with
significant irritation in rabbit skin. However, as a penetration
enhancer, tricaprylin may allow other chemicals to penetrate deeper
into the skin, increasing their concentration so that they may reach
the bloodstream. Ocular exposures of tricaprylin were found to be
only mildly irritating to rabbit eyes. Little or no acute, subchronic,
or chronic oral toxicity was observed in animal studies unless levels
approached a significant percentage of caloric intake. Subcutaneous
injections of tricaprylin in rats over a period of 5 weeks
caused a granulomatous reaction.
Tricaprylin has not been found to be teratogenic in rats, mice, or
hamsters, but some reproductive effects have been seen in rabbits.
Dose-related central nervous system toxicity in dogs has also been
observed.
LD50 (mouse, IP): >27.8 g/kg
LD50 (mouse, IV): 3.7 g/kg
LD50 (mouse, oral): 29.6 g/kg
LD50 (mouse, SC): >27.8 g/kg
LD50 (rat, IP): 0.05 g/kg
LD50 (rat, IV): 4 g/kg
LD50 (rat, oral): 33.3 g/kg
Carcinogenicity
In F344 rats given 10 mL/kg of tricaprylin by gavage daily for 2 years, there is significant increase in the incidence of squamous cell papillomas of the forestomach, compared to controls .
storage
Tricaprylin is classified as a stable compound. It has high stability
against oxidation and is not heat sensitive. Even in hot climates
cooling is not necessary. However, exposure to high temperatures
near the flash point (246℃) should be avoided. Owing to its very
low water content, it is not sensitive to hydrolytic and microbial
splitting. Although polymerization of tricaprylin will not occur, it is
reported to decompose into carbon monoxide and carbon dioxide.
Tricaprylin should be stored in well-closed containers, protected
from light, in a dry place at ambient temperature. High-density
polyethylene, polypropylene, metal (aluminum), and glass are
suitable for packaging. Some plastics, especially those containing
plasticizers, can become brittle or expand in the presence of
tricaprylin. Polystyrene and polyvinyl chloride are not suitable for
its storage. Tricaprylin has a high tendency to migrate, and therefore
care should be taken when selecting seal-closure elastomer material.
Incompatibilities
Tricaprylin is incompatible with strong oxidizing agents.
Regulatory Status
Included in the FDA Inactive Ingredients Database (epidural injections).
Properties of TRIOCTANOIN
| Melting point: | 9-10 °C |
| Boiling point: | 233 °C/1 mmHg (lit.) |
| Density | 0.956 g/mL at 20 °C (lit.) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | n |
| Flash point: | 225 °C |
| storage temp. | -20°C |
| solubility | Miscible with most organic solvents including ethanol
(95%). Captex 8000 is insoluble in water. |
| form | neat |
| form | Liquid |
| color | Colourless |
| Odor | at 100.00?%. odorless |
| Water Solubility | 65mg/L at 20℃ |
| BRN | 1717202 |
| CAS DataBase Reference | 538-23-8(CAS DataBase Reference) |
| EPA Substance Registry System | Glycerol trioctanoate (538-23-8) |
Safety information for TRIOCTANOIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for TRIOCTANOIN
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Tricaprylin CAS 538-23-8View Details
Tricaprylin CAS 538-23-8View Details
538-23-8 -
 Tricaprylin CAS 538-23-8View Details
Tricaprylin CAS 538-23-8View Details
538-23-8 -
 Glyceryl trioctanoate CAS 538-23-8View Details
Glyceryl trioctanoate CAS 538-23-8View Details
538-23-8 -
 Tricaprylin CAS 538-23-8View Details
Tricaprylin CAS 538-23-8View Details
538-23-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1