Trimethyl(phenoxy)silane
Synonym(s):Phenoxy(trimethyl)silane;Phenyl trimethylsilyl ether
- CAS NO.:1529-17-5
- Empirical Formula: C9H14OSi
- Molecular Weight: 166.29
- MDL number: MFCD00053666
- EINECS: 216-211-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
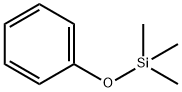
What is Trimethyl(phenoxy)silane?
Chemical properties
Clear colorless liquid
The Uses of Trimethyl(phenoxy)silane
Trimethyl(phenoxy)silane can be used as a catalyst for the Friedel-Crafts reaction, which is an organic reaction that converts alkenes into alcohols or epoxides. It can also reacts with hydrochloric acid to give phenol and chloroform. Trimethyl(phenoxy)silane has been shown to have chemical properties similar to those of phenol, such as viscosity, chemical species and resonance spectroscopy properties. It can react with oxygen in the air to form peroxides, so it should be stored in a cool, dry place away from light.
General Description
Trimethyl(phenoxy)silane is a trimethylsilyl protected alcohol. Direct lateral zincation (DlZn) of trimethyl(phenoxy)silane has been reported. Dielectric studies of phenoxysilane-phenol binary systems has been reported.
Synthesis
General procedure for trimethylsilylation of alcohols with HMDS catalyzed by P2O5/Al2O3. P2O5/Al2O3 (0.1 g) was added to a stirred solution of the alcohol (10 mmol) and HMDS (7.5 mmol) and the mixture was stirred at room temperature for the time specified in Table 2. The reaction was followed by TLC (n-hexane-EtOAc, 9:1). After completion of the reaction, ethyl acetate was added to the reaction mixture and the catalyst was isolated by filtration. It was washed well with ethyl acetate (2 9 5 ml) and then dried at 100°C for 2 h before being used again. The filtered solution was evaporated and purified by passage through a short column of silica gel with n-hexane as eluent (2 x 20 ml). Evaporation of the solvent under reduced pressure gave the pure product Trimethyl(phenoxy)silane, Yield; 89%.
Properties of Trimethyl(phenoxy)silane
| Melting point: | -55 °C |
| Boiling point: | 81 °C23 mm Hg(lit.) |
| Density | 0.92 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | 127 °F |
| storage temp. | Sealed in dry,Room Temperature |
| form | liquid |
| color | Colorless to Light orange to Yellow |
| Specific Gravity | 0.92 |
| λmax | 274nm(Heptane)(lit.) |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 1905943 |
| EPA Substance Registry System | Silane, trimethylphenoxy- (1529-17-5) |
Safety information for Trimethyl(phenoxy)silane
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trimethyl(phenoxy)silane
| InChIKey | OJAJJFGMKAZGRZ-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Trimethyl(phenoxy)silane CAS 1529-17-5View Details
Trimethyl(phenoxy)silane CAS 1529-17-5View Details
1529-17-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
