Trimethylamine hydrochloride
Synonym(s):Trimethylamine hydrochloride;Trimethylammonium chloride
- CAS NO.:593-81-7
- Empirical Formula: C3H10ClN
- Molecular Weight: 95.57
- MDL number: MFCD00012478
- EINECS: 209-810-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
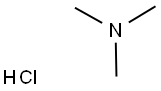
What is Trimethylamine hydrochloride?
Chemical properties
white to slightly cream crystalline powder
The Uses of Trimethylamine hydrochloride
Trimethylamine hydrochloride is used in the manufacture of quaternary ammonium compounds, as insect attractant. It is used as a raw material for the synthesis of choline chloride, quaternary ammonium compounds, ion exchange resins. It acts as cationic starch reagents, phase transfer catalyst, oil field chemicals, warning agent for natural gas and flotation agent.
What are the applications of Application
Trimethylamine hydrochloride(593-81-7) is a precursor to prepare aluminium chloride- trimethylamine hydrochloride ionic liquid, which is commonly used in electrodeposition of aluminum wires. It is also used in the synthesis of trimethylamine gallane, trimethylamine-borane and azido-bridged perovskite-type metal-organic frameworks (MOFs).
Definition
ChEBI: Trimethylamine hydrochloride is a hydrochloride salt formed by reaction of equimolar amounts of trimethylamine and hydrogen chloride. It contains a trimethylammonium.
Preparation
Trimethylamine hydrochloride can be synthesized through the reaction of trimethylamine (TMA) and hydrochloric acid (HCl). The synthesis reaction can be written as follows:
C3H9N+ HCl → C3H10ClN
The following steps are involved in obtaining trimethylamine hydrochloride:
Step 1: Evaporation of hydrochloric acid solution The hydrochloric acid solution is evaporated, first over a free flame, and later on a steam bath. This process results in concentration of the solution, which aids in the formation of trimethylamine hydrochloride crystals.
Step 2: Crystal formation and filtration As the solution becomes more and more concentrated, the trimethylamine hydrochloride crystals begin to form. The crystals are filtered periodically to remove them from the solution.
Step 3: Drying The filtered crystals are dried in an air bath for a few minutes, with a temperature range of 100-110°.
Step 4: Storage The air-dried crystals are then stored in a tightly closed bottle.
Step 5: Centrifugation (optional) If the trimethylamine hydrochloride crystals are centrifuged, the product is obtained pure and dry.
Overall, four runs yield an average of 710 g of pure trimethylamine hydrochloride and 82 g of slightly yellow-tinted product. This yield is equivalent to 89% of the theoretical amount based on ammonium chloride. The slight yellow coloration results from the evaporation of the last traces of solution.
DOI: 10.15227/orgsyn.001.0079
Flammability and Explosibility
Non flammable
Purification Methods
The salt crystallises from CHCl3, EtOH or n-propanol, and is dried under vacuum. It also crystallises from *benzene/MeOH, MeOH/diethyl ether and is dried under vacuum over paraffin wax and H2SO4. It is kept over P2O5 as it is hygroscopic.[Beilstein 4 H 262, 4 I 419, 4 IV 138.]
Properties of Trimethylamine hydrochloride
| Melting point: | 283-284 °C (dec.)(lit.) |
| Boiling point: | 132.32°C (rough estimate) |
| Density | 0.9337 (rough estimate) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.4413 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | SOLUBLE |
| form | Crystalline Powder |
| color | White to slightly cream |
| PH | 5 (100g/l, H2O, 20℃) |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,9710 |
| BRN | 3905063 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 593-81-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Methanamine,n,n-dimethyl-,hydrochloride(593-81-7) |
| EPA Substance Registry System | Trimethylamine hydrochloride (593-81-7) |
Safety information for Trimethylamine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Trimethylamine hydrochloride
| InChIKey | SZYJELPVAFJOGJ-UHFFFAOYSA-N |
Trimethylamine hydrochloride manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Trimethylamine Hydrochloride 593-81-7 99%View Details
Trimethylamine Hydrochloride 593-81-7 99%View Details
593-81-7 -
 Tri Methyl Amine HCL 99%View Details
Tri Methyl Amine HCL 99%View Details -
 Trimethylammonium chloride CAS 593-81-7View Details
Trimethylammonium chloride CAS 593-81-7View Details
593-81-7 -
 Trimethylamine hydrochloride CAS 593-81-7View Details
Trimethylamine hydrochloride CAS 593-81-7View Details
593-81-7 -
 Trimethylamine Hydrochloride CAS 593-81-7View Details
Trimethylamine Hydrochloride CAS 593-81-7View Details
593-81-7 -
 Trimethylamine hydrochloride CAS 593-81-7View Details
Trimethylamine hydrochloride CAS 593-81-7View Details
593-81-7 -
 Tri Methyl Amine HydrochlorideView Details
Tri Methyl Amine HydrochlorideView Details
593-81-7 -
 Trimethylamine HydrochlorideView Details
Trimethylamine HydrochlorideView Details
593-81-7
