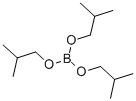
Triisopropyl borate
Synonym(s):Boric acid triisopropyl ester;Boron isopropoxide;Triisopropoxyborane;Triisopropyl borate
- CAS NO.:5419-55-6
- Empirical Formula: C9H21BO3
- Molecular Weight: 188.07
- MDL number: MFCD00008872
- EINECS: 226-529-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-10 11:22:48
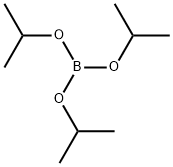
What is Triisopropyl borate?
Chemical properties
clear colourless liquid
The Uses of Triisopropyl borate
Triisopropyl borate is used as reagent in Pd-catalyzed coupling reaction with aryl halides such as Suzuki reaction. It is used as a reagent for the preparation of the boronic acids and esters; as a Lewis acid catalyst and involved in the ortho-borylation of 1-substituted naphthalenes. Furthermore, it plays an important role as a catalyst for the production of resins, waxes, paints and varnishes.
General Description
A colorless liquid. Flash point 93°F. Less dense than water and insoluble in water. Vapors heavier than air. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Borates, such as Triisopropyl borate, behave similarly to esters in that they react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters/borates with alkali metals and hydrides.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Properties of Triisopropyl borate
| Melting point: | -59 °C |
| Boiling point: | 139-141 °C (lit.) |
| Density | 0.815 g/mL at 25 °C (lit.) |
| vapor pressure | 76 mm Hg ( 75 °C) |
| refractive index | n |
| Flash point: | 62.6 °F |
| storage temp. | Store below +30°C. |
| solubility | Miscible with ether, ethanol, isopropanol and benzene. |
| form | Liquid |
| appearance | Liquid |
| color | Clear colorless |
| Specific Gravity | 0.815 |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 1701469 |
| CAS DataBase Reference | 5419-55-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Boric acid (h3bo3), tris(1-methylethyl) ester(5419-55-6) |
| EPA Substance Registry System | Triisopropyl orthoborate (5419-55-6) |
Safety information for Triisopropyl borate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. |
Computed Descriptors for Triisopropyl borate
Triisopropyl borate manufacturer
ASM Organics
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran

You may like
-
 5419-55-6View Details
5419-55-6View Details
5419-55-6 -
 Tri isopropyl borate (TIPB)View Details
Tri isopropyl borate (TIPB)View Details
5419-55-6 -
 Triisopropyl borate 97% CAS 5419-55-6View Details
Triisopropyl borate 97% CAS 5419-55-6View Details
5419-55-6 -
 Triisopropyl borate CAS 5419-55-6View Details
Triisopropyl borate CAS 5419-55-6View Details
5419-55-6 -
 Triisopropyl Borate CAS 5419-55-6View Details
Triisopropyl Borate CAS 5419-55-6View Details
5419-55-6 -
 5419-55-6 99%View Details
5419-55-6 99%View Details
5419-55-6 -
 Triisopropyl borate CAS 5419-55-6View Details
Triisopropyl borate CAS 5419-55-6View Details
5419-55-6 -
 Triisopropyl borate CAS 5419-55-6View Details
Triisopropyl borate CAS 5419-55-6View Details
5419-55-6
