trichloronaphthalene
- CAS NO.:1321-65-9
- Empirical Formula: C10H5Cl3
- Molecular Weight: 231.5057
- EINECS: 215-321-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
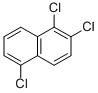
What is trichloronaphthalene?
Chemical properties
The chlorinated naphthalenes in which one or more hydrogen atoms have been replaced by chlorine to form wax-like substances, beginning with monochloronaphthalene and going on to the octachlor derivatives. Their physical states vary from mobile liquids to waxysolids depending on the degree of chlorination; freezing/ melting points of the pure compounds range from 17C for 1-chloronaphthalene to 198C for 1,2,3,4- tetrachloronaphthalene. 1-Chloro-isomer: Hazard identification (based on NFPA-704 M Rating System): Health 2, flammability 1, reactivity 0. 2-Chloro-isomer:
The Uses of trichloronaphthalene
Wire coating, electrical insulations.
The Uses of trichloronaphthalene
Electric wire insulation; lubricants.
General Description
Colorless to pale-yellow solid with an aromatic odor. Mp: 93°C; bp: 304-354°C. Used in lubricants and in the manufacture of insulation for electrical wire. Presents an environmental danger. If released into the environment, bioaccumulation takes place in fish. Will persist in the environment causing long-term adverse effects. The halowaxes are technical-grade chlorinated naphthalenes containing trichloronaphthalene in its various isomers together with (mainly) tetrachloro- pentachloro- and hexa-chloronapthalenes in their various isomers.
Reactivity Profile
trichloronaphthalene is non-flammable, but combustible. Gives off irritating or toxic gases in a fire. Incompatible with strong oxidizing agents. Reacts violently with liquid oxygen. Also can react violently with aluminum and with bases.
Hazard
A poison. Liver damage and chloracne.
Health Hazard
Trichloronaphthalene is moderately toxic to the liver.Industrial exposure to trichloronaphthalene (usually mixed with tetrachloronaphthalene) has been relatively free of untoward effects compared with the more highly chlorinated naphthalenes.The higher-chlorinated naphthalenes show a much greater toxicity.
Safety Profile
A poison. The chlorinated naphthalenes have toxic effects on the skin and liver. See also CHLORINATED HYDROCARBONS, ALIPHATIC; and POLYCHLORINATED BIPHENYLS.
Potential Exposure
Industrial exposure from individual chlorinated naphthalenes is rarely encountered; rather it usually occurs from mixtures of two or more Chlorinated naphthalenes. Due to their stability, thermoplasticity, and nonflammability, these compounds enjoy wide industrial application. These compounds are used in the production of electric condensers; in the insulation of electric cables and wires; as additives to extreme pressure lubricants; as supports for storage batteries; and as a coating in foundry use. octachloro-: Used as a fireproof and waterproof additive and lubricant additive. Pentachloro-: Used in electric wire insulation and in additives to special lubricants. tetrachloro-: Used in electrical insulating materials and as an additive in cutting oils. trichloro-: Used in lubricants and in the manufacture of insulation for electrical wire. Because of the possible potentiation of the toxicity of higher Chlorinated naphthalenes by ethanol and carbon tetrachloride, individuals who ingest enough alcohol to result in liver dysfunction would be a special group at risk. Individuals, e.g., analytical and synthetic chemists, mechanics and cleaners, who are routinely exposed to carbon tetrachloride or other hepatotoxic chemicals would also be at a greater risk than a population without such exposure. Individuals involved in the manufacture, utilization, or disposal of polychlorinated naphthalenes would be expected to have higher levels of exposure than the general population.
Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
All are incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Keep away from heat. Penta- is also incompatible with acids, alkalis.
Waste Disposal
High-temperature incineration with flue gas scrubbing. Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
Properties of trichloronaphthalene
| Melting point: | 92.8°C |
| Boiling point: | 301.08°C (rough estimate) |
| Density | 1.5800 |
| refractive index | 1.6030 (estimate) |
| form | solid |
| color | White |
| CAS DataBase Reference | 1321-65-9 |
| EPA Substance Registry System | Trichloronaphthalene (1321-65-9) |
Safety information for trichloronaphthalene
Computed Descriptors for trichloronaphthalene
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8