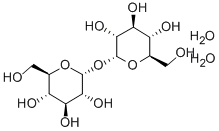
Trehalose
Synonym(s):α,α-Trehalose;α-D -Glucopyranosyl-α-D -glucopyranoside;D -(+)-Trehalose dihydrate
- CAS NO.:99-20-7
- Empirical Formula: C12H22O11
- Molecular Weight: 342.3
- MDL number: MFCD00006628
- EINECS: 202-739-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18

What is Trehalose?
Description
Plants and animals use trehalose for energy and to stabilize themselves against extreme conditions. Mushrooms, honey, yeast, lobster, and shrimp are sources of trehalose in the human diet. Recent studies on mouse models indicate that trehalose might reduce the symptoms of Huntington''s disease.
Chemical properties
white to off-white crystalline powder
Chemical properties
Trehalose occurs as virtually odorless, white or almost white crystals with a sweet taste (approximately 45% of the sweetness of sucrose).
The Uses of Trehalose
trehalose is a humectant and moisturizer, it helps bind water in the skin and increase the skin’s moisture content. It is a naturally occurring plant sugar.
The Uses of Trehalose
D-(+)-Trehalose can be used for the effects of sucrose on blood avidity in mosquitoes.
What are the applications of Application
D-(+)-Trehalose Anhydrous is formed by the bonding of two reducing aldehyde groups
Definition
ChEBI: A trehalose in which both glucose residues have alpha-configuration at the anomeric carbon.
Production Methods
Trehalose is prepared from liquefied starch by a multistep enzymatic process. The commercial product is the dihydrate.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Trehalose is used for the lyoprotection of therapeutic proteins, particularly for parenteral administration. Other pharmaceutically relevant applications include use as an excipient for diagnostic assay tablets; for stabilization during the freeze–thaw and lyophilization of liposomes; and for stabilization of blood cells, cosmetics, and monoclonal antibodies. Trehalose may also be used in formulations for topical application.
Safety
Trehalose is used in cosmetics, foods, and parenteral and
nonparenteral pharmaceutical formulations. It is generally regarded
as a relatively nontoxic and nonirritant material when used as an
excipient.
In the gut, trehalose is rapidly metabolized to glucose by the
specific enzyme trehalase. A small minority of the population
exhibits a primary (hereditary) or secondary (acquired) trehalase
deficiency and thus may experience intestinal discomfort after
ingestion of excessive amounts of trehalose owing to the osmotic
activity of undigested trehalose in the gut. However, smaller
amounts of trehalose are tolerated by such individuals without
any symptoms.
Trehalose is used as a sweetener and is reported to have
substantially less cariogenic potential than sucrose.
LD50 (dog, IV): >1 g/kg
LD50 (dog, oral): >5 g/kg
LD50 (mouse, IV): >1 g/kg
LD50 (mouse, oral): >5 g/kg
LD50 (rat, IV): >1 g/kg
LD50 (rat, oral): >5 g/kg
Storage
Trehalose is a relatively stable material. At 60°C for 5 hours it loses
not more than 1.5% w/w of water (the dihydrate water of crystallization is retained). Open stored powder may liquefy at high
relative humidity (≥90%).
Trehalose should be stored in a cool, dry place in a well-sealed
container.
Incompatibilities
Trehalose is incompatible with strong oxidizing agents, especially in the presence of heat.
Regulatory Status
GRAS listed. In the UK trehalose may be used in certain food applications. Included in parenteral and nonparenteral investigational formulations.
Properties of Trehalose
| Melting point: | 203 °C |
| Boiling point: | 397.76°C (rough estimate) |
| Density | 1.5800 |
| vapor pressure | 0.001Pa at 25℃ |
| RTECS | LZ5776770 |
| refractive index | 197 ° (C=7, H2O) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Soluble in water; very slightly soluble in ethanol (95%);
practically insoluble in ether. |
| form | Powder |
| pka | 12.53±0.70(Predicted) |
| color | White to Off-white |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| Merck | 14,9580 |
| CAS DataBase Reference | 99-20-7(CAS DataBase Reference) |
| EPA Substance Registry System | Trehalose (99-20-7) |
Safety information for Trehalose
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trehalose
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![TREHALOSE, ALPHA-D-, [14C(U)]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB7481536.gif)

You may like
-
 D-(+)-Trehalose, anhydrous CAS 99-20-7View Details
D-(+)-Trehalose, anhydrous CAS 99-20-7View Details
99-20-7 -
 D-(+)-Trehalose Anhydrous CAS 99-20-7View Details
D-(+)-Trehalose Anhydrous CAS 99-20-7View Details
99-20-7 -
 D-Trehalose 95 % CAS 99-20-7View Details
D-Trehalose 95 % CAS 99-20-7View Details
99-20-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
