TRANS-STILBENE
- CAS NO.:103-30-0
- Empirical Formula: C14H12
- Molecular Weight: 180.25
- MDL number: MFCD00064300
- EINECS: 203-098-5
- Update Date: 2025-12-16 16:15:04
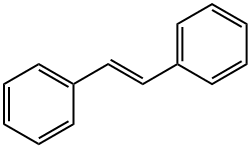
What is TRANS-STILBENE?
Chemical properties
off-white crystalline powder
The Uses of TRANS-STILBENE
trans-Stilbene is used in the manufacturing of dye lasers, optical brighteners, non-steroidal synthetic estrogens such as diethylstilbestrol, fosfestrol and dienestrol. It is used as a phosphor and a scintillator. It is also involved in transition metal catalyzed asymmetric epoxidation and dihydroxylation. Further, it is used as raw material in organic synthesis.
The Uses of TRANS-STILBENE
Manufacture of dyes and optical bleaches, crystals are used as phosphors and scintillators.
The Uses of TRANS-STILBENE
Commonly employed in transition metal catalyzed asymmetric epoxidation and dihydroxylation.
Definition
ChEBI: Stilbene is an olefinic compound and a member of benzenes.
General Description
Off-white crystals. Melting point of 122-124°C. Shows blue fluorescence.
Air & Water Reactions
Sensitive to air and light. Insoluble in water.
Reactivity Profile
TRANS-STILBENE may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release gaseous hydrogen.
Fire Hazard
Flash point data for TRANS-STILBENE are not available, however TRANS-STILBENE is probably combustible.
Environmental Fate
Trans-1,2-Diphenylethylene's production and use in the manufacture of dyes and optical bleaches, and as phosphors and scintillators may result in its release to the environment. If released to air, a vapor pressure of 8.81X10-4 mm Hg at 25 °C indicates trans-1,2-diphenylethylene will exist solely as a vapor in the ambient atmosphere. Vapor-phase trans-1,2-diphenylethylene will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals and ozone molecules. The half-life for the reaction in air with hydroxyl radicals is estimated to be 6 hours. The half-life for the reaction in air with ozone is estimated to be 1 hour. If released to soil, trans-1,2-diphenylethylene is expected to have no mobility based upon an estimated Koc of 9,850. Volatilization from moist soil surfaces may be an important fate process based upon an estimated Henry's Law constant of 7.2X10-4 atm-cu m/mole; however, adsorption to soil may attenuate this process.
Trans-1,2-Diphenylethylene is not expected to volatilize from dry soil surfaces based upon its vapor pressure. If released into water, trans-1,2-diphenylethylene is expected to adsorb to suspended solids and sediment based upon its estimated Koc. Volatilization from water surfaces may be an important fate process based upon this compound's estimated Henry's Law constant. Estimated volatilization half-lives for a model river and model lake are 2 and 140 hours, respectively. However, volatilization from water surfaces is expected to be attenuated by adsorption to suspended solids and sediment. The estimated volatilation half-life from a model pond is 90 days if adsorption is considered. An estimated BCF of 1,000 suggests the potential for bioconcentration in aquatic organisms is very high. Occupational exposure to trans-1,2-diphenylethylene may occur through inhalation of dust particles and dermal contact with this compound at workplaces where it is produced or used. Limited monitoring data indicate that non-occupational exposure can occur from the ingestion of contaminated drinking water. (SRC)
Purification Methods
Purify it by vacuum distillation. (The final product contains about 1% of the cis isomer.) Crystallise it from EtOH. It has also been purified by zone melting. The styphnate (see next entry) has m 142o. [Lewis et al. J Am Chem Soc 107 203 1985, Bollucci et al. J Am Chem Soc 109 515 1987, Saltiel J Phys Chem 91 2755 1987, Beilstein 5 IV 2156.]
Properties of TRANS-STILBENE
| Melting point: | 123-125 °C(lit.) |
| Boiling point: | 305-307 °C744 mm Hg(lit.) |
| Density | 0.97 g/mL at 25 °C(lit.) |
| refractive index | 1.6230 |
| Flash point: | 305-307°C |
| storage temp. | Store below +30°C. |
| solubility | 0.00029g/l |
| form | Crystals or Crystalline Powder |
| Specific Gravity | 0.97 |
| color | White to slightly beige |
| Water Solubility | Soluble in benzene and ether. Insoluble in water. |
| Merck | 14,8817 |
| BRN | 1616740 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 103-30-0(CAS DataBase Reference) |
| EPA Substance Registry System | trans-Stilbene (103-30-0) |
Safety information for TRANS-STILBENE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for TRANS-STILBENE
| InChIKey | PJANXHGTPQOBST-VAWYXSNFSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 103-30-0 trans-Stilbene 98%View Details
103-30-0 trans-Stilbene 98%View Details
103-30-0 -
 trans-Stilbene, 96% CAS 103-30-0View Details
trans-Stilbene, 96% CAS 103-30-0View Details
103-30-0 -
 Trans-stilbene 95% CAS 103-30-0View Details
Trans-stilbene 95% CAS 103-30-0View Details
103-30-0 -
 trans-Stilbene CAS 103-30-0View Details
trans-Stilbene CAS 103-30-0View Details
103-30-0 -
 trans-Stilbene CAS 103-30-0View Details
trans-Stilbene CAS 103-30-0View Details
103-30-0 -
 trans-Stilbene CAS 103-30-0View Details
trans-Stilbene CAS 103-30-0View Details
103-30-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
