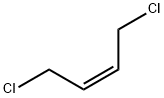
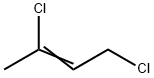
TRANS-1,4-DICHLORO-2-BUTENE
- CAS NO.:764-41-0
- Empirical Formula: C4H6Cl2
- Molecular Weight: 125
- MDL number: MFCD00000988
- EINECS: 212-121-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is TRANS-1,4-DICHLORO-2-BUTENE?
Description
The 1,4-dichloro-2-butenes are colorlessliquids. Some have a distinct odor.(cis-isomer): Molecular weight = 125; Boilingpoint=153℃; Freezing/Melting point = 248℃. Explosivelimits: LEL = 1.5%; UEL = 4.0%. Reacts slowly withwater.(trans-isomer): This material has a sweet, pungent odor.Boiling point = 155.5℃; Freezing/Melting point 5 1-3℃;Flash point = 53℃. The explosive limits for the trans-isomer are LEL = 1.5%; UEL = 4%. Hazard Identification(based on NFPA-704 M Rating System): Health 2,Flammability 2, Reactivity 0. Insoluble in water. The transisomer, only, appears on the EHS list.
Chemical properties
The 1,4-dichloro-2-butenes are colorless liquids. Some have a distinct odor. (cis-isomer)
The Uses of TRANS-1,4-DICHLORO-2-BUTENE
1,?4-?Dichloro-?2-?butene is an organic reagent used in the preparation of heterocyclic compounds. Used in the synthesis of γ-turn peptidomimetic scaffold neuroprotectives as a JNK3 allosteric ligand.Environmental toxin on US EPA Toxic Release Inventory list (TRI) list.
Definition
ChEBI: Trans-1,4-Dichlorobutene is an organochlorine compound.
General Description
A clear colorless liquid. Burns, though may be difficult to ignite. Corrosive to tissue. Denser than water and insoluble in water. Vapors heavier than air. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Reacts slowly with water to form hydrochloric acid. Insoluble in water.
Reactivity Profile
Halogenated unsaturated aliphatic compounds, such as TRANS-1,4-DICHLORO-2-BUTENE, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Low molecular weight haloalkanes are highly flammable and can react with some metals to form dangerous products. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides.
Health Hazard
Inhalation of vapor irritates nose and throat. Contact with eyes causes intense irritation and tears. Contact of liquid with skin causes severe blistering and dermatitis. Ingestion causes severe irritation of mouth and stomach.
Fire Hazard
Special Hazards of Combustion Products: Decomposition vapors contain phosgene and hydrogen chloride gases; both are toxic and irritating.
Chemical Reactivity
Reactivity with Water Reacts slowly to form hydrochloric acid; Reactivity with Common Materials: Corrodes metal when wet; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. Poison by ingestion, inhalation, and intravenous routes. Moderately toxic by skin contact. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. A severe skin and eye irritant. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, ALIPHATIC .
Potential Exposure
DC occurs as a by-product in chloroprene manufacture and may be used as a chemical intermediate
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions,including resuscitation mask) if breathing has stopped andCPR if heart action has stopped. Transfer promptly to amedical facility. When this chemical has been swallowed,get medical attention. If victim is conscious, administerwater or milk. Do not induce vomiting.
Storage
(1) Color Code—Red (cis-isomer): FlammabilityHazard: Store in a flammable liquid storage area orapproved cabinet away from ignition sources and corrosiveand reactive materials. (2) Color Code—White: Corrosiveor Contact Hazard; Store separately in a corrosion-resistantlocation. Prior to working with this chemical you should betrained on its proper handling and storage. Before enteringconfined space where this chemical may be present, checkto make sure that an explosive concentration does not exist.Store in tightly closed containers in a cool, well-ventilatedarea away from oxidizers. Where possible, automaticallypump liquid from drums or other storage containers to process containers. A regulated, marked area should be established where this chemical is handled, used, or stored incompliance with OSHA Standard 1910.1045.
Shipping
UN3384 Toxic by inhalation liquid, flammable, n. o.s. with an LC50 ≦ 1000 ml/m3 and saturated vapor concentration ≧to 10 LC50, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid, Technical Name Required, Inhalation Hazard Zone B. UN2920 Corrosive liquids, flammable, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, 3-Flammable liquid. The code “D” identifies proper shipping names which are appropriate for describing materials for domestic transportation but may be inappropriate for international transportation under the provisions of international regulations, e.g., IMO, ICAO). An alternate proper shipping name may be selected when either domestic or international transportation is involved
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. (cis-isomer): Reacts slowly with water forming hydrogen chloride. Attacks metals and may form other, more dangerous materials; attacks some plastics.
Waste Disposal
High-temperature incineration with hydrochloric acid scrubbing. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of TRANS-1,4-DICHLORO-2-BUTENE
| Melting point: | 1-3 °C(lit.) |
| Boiling point: | 74-76 °C40 mm Hg(lit.) |
| Density | 1.183 g/mL at 25 °C(lit.) |
| vapor pressure | 10 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 129 °F |
| storage temp. | 2-8°C |
| Odor | Characteristic; sweet, pungent. |
| CAS DataBase Reference | 764-41-0(CAS DataBase Reference) |
| EPA Substance Registry System | 1,4-Dichloro-2-butene (764-41-0) |
Safety information for TRANS-1,4-DICHLORO-2-BUTENE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H314:Skin corrosion/irritation H330:Acute toxicity,inhalation H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for TRANS-1,4-DICHLORO-2-BUTENE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 764-41-0 Trans-1,4-Dichloro-2-butene 98%View Details
764-41-0 Trans-1,4-Dichloro-2-butene 98%View Details
764-41-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
