trans-1,3-Dichloropropene
- CAS NO.:10061-02-6
- Empirical Formula: C3H4Cl2
- Molecular Weight: 110.97
- MDL number: MFCD00000985
- EINECS: 208-826-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32

What is trans-1,3-Dichloropropene?
Description
Trans-1,3-dichloropropene appears as a clear colorless liquid with chloroform odor. Flash point 95 °F. Density 1.225 g/cm3 and insoluble in water. Hence sinks in water. A strong irritant. Used as a soil fumigant. It is used as an intermediate in the manufacture of 3,3-dichloro-1-propene and other pesticides.
Chemical properties
colourless liquid
Chemical properties
1,3-Dichloropropene is a colorless to strawcolored liquid. Sharp, sweet, irritating, chloroform-like odor.
Physical properties
Colorless to pale yellow liquid with a chloroform-like odor. Evaporates quickly when spilled.
The Uses of trans-1,3-Dichloropropene
The isomer mixture is used as a soil fumigant and a nematocide.
Definition
ChEBI: (E)-1,3-dichloropropene is a 1,3-dichloropropene with a (E)-configuration. It has a role as a fumigant. It is a 1,3-dichloropropene and a chloroalkene. It derives from a hydride of a propene.
General Description
A clear colorless liquid with chloroform odor . Flash point 95°. Density 1.225 g / cm3 and insoluble in water. Hence sinks in water. A strong irritant. Used as a soil fumigant.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
trans-1,3-Dichloropropene reacts with aluminum, aluminum alloys, with other active metals and with some metal salts and halogens. Can react vigorously with oxidizing materials. .
Fire Hazard
Flash point data for trans-1,3-Dichloropropene are not available, however literature indicates that trans-1,3-Dichloropropene is flammable.
Potential Exposure
Used as a soil fumigant prior to planting crops, such as cotton, sugar beet, potatoes; used in combinations with dichloropropanes as a soil fumigant. Workers engaged in manufacture, formulation and application of this soil fumigant and nematocide.
Environmental Fate
Biological. The isomeric mixture showed significant degradation with gradual adaptation
in a static-culture flask-screening test (settled domestic wastewater inoculum) conducted
at 25°C. At concentrations of 5 and 10 mg/L, percent losses after 4 weeks of
incubation were 85 and 84, respectively. Ten days into the incubation study, 7–19% was
lost due to volatilization (Tabak et al., 1981).
Chemical/Physical. Hydrolysis in distilled water at 25°C produced trans-3-chloro-2-
propen-1-ol and hydrochloric acid. The reported half-life for this reaction is only 2 days (Kollig, 1993; Milano et al., 1988). trans-1,3-Dichloropropylene was reported to hydrolyze
to 3-chloro-2-propen-1-ol and can be biologically oxidized to 3-chloropropenoic acid
which is further oxidized to formylacetic acid. Decarboxylation of this compound yields
carbon dioxide (Connors et al., 1990). Chloroacetaldehyde, formyl chloride and chloroacetic
acid were formed from the ozonation of dichloropropylene at approximately 23°C
and 730 mmHg. Chloroacetaldehyde and formyl chloride also formed from the reaction
of dichloropropylene and hydroxyl radicals (Tuazon et al., 1984).
The evaporation half-life of trans-1,3-dichloropropylene (1 mg/L) from water at 25°C
using a shallow-pitch propeller stirrer at 200 rpm at an average depth of 6.5 cm is 24.6
minutes (Dilling, 1977).
Emits chlorinated acids when incinerated. Incomplete combustion may release toxic
phosgene (Sittig, 1985).
Solubility in water
Miscible with acetone, benzene, carbon tetrachloride, heptane, methanol (Worthing and Hance, 1991), methylene chloride, chloroform, trichloroethylene, and 1,1,2,2-tetrachloroethane.
Shipping
UN2047 Dichloropropene, Hazard Class: 3; Labels: 3-Flammable liquid.
Incompatibilities
Vapor may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. May accumulate static electrical charges, and may cause ignition of its vapors. Incompatible with strong acids; oxidizers, aluminum or magnesium compounds; aliphatic amines; alkanolamines, alkaline materials; halogens, or corrosives. Note: Epichlorohydrin may be added as a stabilizer.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of trans-1,3-Dichloropropene
| Boiling point: | 97-112 °C(lit.) |
| Density | 1.198 g/mL at 25 °C(lit.) |
| vapor pressure | 79.1 at 50 °C, 523.5 at 100 °C (Wilding et al., 2002) |
| refractive index | n |
| Flash point: | 78 °F |
| storage temp. | 0-6°C
|
| solubility | Miscible with acetone, benzene, carbon tetrachloride, heptane, methanol (Worthing and Hance,
1991), methylene chloride, chloroform, trichloroethylene, and 1,1,2,2-tetrachloroethane. |
| form | neat |
| Specific Gravity | 1.217 |
| color | Colorless to Light yellow to Light orange |
| Water Solubility | 2.8g/L(20 ºC) |
| Merck | 14,3075 |
| Henry's Law Constant | 1.3 x 10-3 atm?m3/mol (Pankow and Rosen, 1988)
0.793 x 10-3 atm?m3/mol at 20 °C, 1.87 at 40 °C (static headspace-GC, Kim et al., 2003) |
| Exposure limits | ACGIH TLV: TWA 1 ppm for cis and trans isomers (adopted). |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents, aluminium, aluminium alloys, some metal salts and halogens. May be light sensitive. |
| CAS DataBase Reference | 10061-02-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Propene, 1,3-dichloro-, (E)-(10061-02-6) |
| EPA Substance Registry System | trans-1,3-Dichloropropene (10061-02-6) |
Safety information for trans-1,3-Dichloropropene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for trans-1,3-Dichloropropene
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
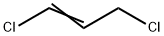



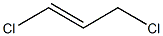
You may like
-
 trans-1,3-Dichloropropene CAS 10061-02-6View Details
trans-1,3-Dichloropropene CAS 10061-02-6View Details
10061-02-6 -
 Trans-1,3-dichloropropene 98% CAS 10061-02-6View Details
Trans-1,3-dichloropropene 98% CAS 10061-02-6View Details
10061-02-6 -
 trans-1,3-Dichloropropene CAS 10061-02-6View Details
trans-1,3-Dichloropropene CAS 10061-02-6View Details
10061-02-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1