1,3-Dichloropropene
- CAS NO.:542-75-6
- Empirical Formula: C3H4Cl2
- Molecular Weight: 110.97
- MDL number: MFCD00000985
- EINECS: 208-826-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
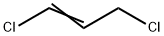
What is 1,3-Dichloropropene?
Description
This nematocide is used as a sol fumigant prior to crop cultivation. Mainly farmers and process operators employed at pesticide plants are exposed.
Chemical properties
clear yellowish to brownish liquid
Chemical properties
1,3-Dichloropropene is a colorless to strawcolored liquid. Sharp, sweet, irritating, chloroform-like odor.
The Uses of 1,3-Dichloropropene
1,3-Dichloropropene (a technical-grade mixture of the cis-and transisomers) is used as a preplanting fumigant, mainly for the control of nematodes affecting the roots of plants, selected plant diseases, garden centipedes, wireworms, and weeds; as a solvent; and as an intermediate in the manufacture of 3,3-dichloro-1-propene and other pesticides. It is registered for use on all vegetable, fruit, and nut crops, all forage crops, tobacco, all fiber crops, and all nursery crops (EPA 1998). In Hawaii, 1,3-dichloropropene is used to control nematodes on pineapples at planting (Albrecht 1987). In 2009, three products containing 1,3-dichloropropene as an active ingredient were registered for restricted, non-residential use in the United States (EPA 2009). No products containing 1,3-dichloropropene are registered for use by homeowners (EPA 1998).
The Uses of 1,3-Dichloropropene
1,3-Dichloropropene is a soil fumigant /nematicide used mainly for the pre-plant control of most species of nematodes and soil insect pests in citrus, pineapples, deciduous fruits and nuts, grapes, berries and field and nursery crops. The product contains equal amounts of E- and Z-isomer. The Z-isomer is more biologically active than the E-isomer.
The Uses of 1,3-Dichloropropene
Widely used as a preplanting soil fumigant for the control of nematodes
Production Methods
1,3-DCP is produced either by high-temperature chlorination
of propylene or from 1,3-dichloro-2-propanol by dehydration
with phosphoryl chloride or phosphorus pentoxide in
benzene. All commercial preparations of 1,3-DCP are mixtures
of the cis- and trans-isomers.
According to SRI Consulting, Dow AgroSciences LLC
(Freeport, Texas) is the only current manufacturer of 1,3-
DCP. Active registrants of 1,3-DCP pesticide formulations
include Dow AgroSciences LLC (Indianapolis, Indiana),Soil Chemicals Corporation (Hollister, California), and
Trical (Hollister, California).
General Description
A clear colorless liquid. Flash point 95°F. Denser (at 10.2 lb / gal) than water and insoluble in water. Vapors are heavier than air. Used to make other chemicals and as soil fumigant.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
1,3-Dichloropropene reacts vigorously with oxidizing materials. Reacts with aluminum, active metals, and halogenated compounds. Also reacts with acids and thiocyanates. Corrosive to magnesium, magnesium alloys and aluminum alloys. Incompatible with some metal salts .
Health Hazard
VAPOR: Irritating to eyes, nose and throat. LIQUID: Will burn skin and eyes. Harmful if swallowed.
Fire Hazard
FLAMMABLE. POISONOUS GASES ARE PRODUCED IN FIRE. Flashback along vapor trail may occur. Vapor may explode if ignited in an enclosed area. Toxic and irritating gases may be generated.
Agricultural Uses
Soil fumigant, Nematicide: This chemical is also used in combinations with dichloropropanes as a soil fumigant to kill nematodes, insects and fungus on cotton, potatoes, tobacco, sugar beets, vegetables, grain, citrus planting sites, deciduous fruit and nut-tree planting sites, and ornamental trees and floral sites. Top four applications in California are on sweet potatoes, carrots, wine grapes and outdoor propagation nurseries. It is used on a wide variety of crops. Not approved for use in EU countries. Actively registered in the U.S.
Trade name
DURHAM NEMATOCIDE®[C]; FUMAZONE®[C]; NEMAGON®[C]; NEMEX®; PROKILL NEMATOCIDE®[C]; TELONE®; TELONE II®; TELONE II-B®; TELONE® EC DRIP; VIDDEN D®; VORLEX®
Contact allergens
This nematocide is used as a soil fumigant prior to crop cultivation. Farmers and process operators employed at pesticide plants are mainly exposed
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Poison by ingestion and intraperitoneal routes. Moderately toxic by skin contact. Mildly toxic by inhalation. A strong irritant. Mutation data reported. A pesticide. A flammable liquid and dangerous fire hazard when exposed to heat, flame, or oxidzers. Reacts vigorously with oxidizing materials. To fight fire, use water, foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of Cl-. See also ALLYL COMPOUNDS and CHLORIDES.
Potential Exposure
Used as a soil fumigant prior to planting crops, such as cotton, sugar beet, potatoes; used in combinations with dichloropropanes as a soil fumigant. Workers engaged in manufacture, formulation and application of this soil fumigant and nematocide.
Carcinogenicity
Technical-grade 1,3-dichloropropene (co ntaining 1.0% epichlorohydrin as a stabilizer) is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. The technical-grade 1,3-dichloropropene used in the cancer studies in experimental animals was a mixture of cis- and trans-isomers and varied in purity and the stabilizer used (see Properties).
Metabolic pathway
Degradation of 14C-1,3-dichloropropene in aerobic soils in the dark at 25℃ at concentration of approximately 100 μg g-1 results in the formation of 3-chloroallyl alchohol, 3-chloroacrylic acid, numerous minor carboxylic acid metabolites, and carbon dioxide. In addition, there is extensive incorporation of 14C labeled material into the soil organic matter in the soils. By a specific bacterium (Pseudomonas cichorii 170) which is isolated from soil, 1,3-dichloropropene is degraded in different pathways from those of soil degradation. The terminal degradate by this bacterium is acetoaldehye which undergoes mineralization.
Shipping
UN2047 Dichloropropene, Hazard Class: 3; Labels: 3-Flammable liquid.
Degradation
E-1,3-Dichloropropene (1) and Z-1,3-dichloropropene (2) degraded
rapidly in aqueous solution (independent of pH) under both dark and
light exposed conditions (25 °C), with DTW values of ca. 1-6 days. Hydrolytic
dechlorination reactions yielded E- and Z-3-chloroallyl alcohol (3,4)
and hydrochloric acid (5) as the major products. Further oxidation of
3 and 4 yielded E- and Z-3-chloroacrylic acid (6, 7) as minor products
(McCall, 1987; Milano et al., 1988; Batzer and Yoder, 1995).
1,3-Dichloropropene degraded in the vapour phase (with ozone and
hydroxyl radicals) to yield chloroacetaldehyde (8), formyl chloride (9) and
chloroacetic acid (10 (Tuazon et al., 1984). The estimated air photolysis
DT50 was ca. 7-12 hours (Roby and Melichar, 1997).
Toxicity evaluation
In water hydrolyzes to 3-chloropropenol and chloride ion. Both isomers of 3-chloropropenol are toxic but less so than the parent compound.
Incompatibilities
Vapor may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. May accumulate static electrical charges, and may cause ignition of its vapors. Incompatible with strong acids; oxidizers, aluminum or magnesium compounds; aliphatic amines; alkanolamines, alkaline materials; halogens, or corrosives. Note: Epichlorohydrin may be added as a stabilizer.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of 1,3-Dichloropropene
| Melting point: | -60 °C |
| Boiling point: | 97-112 °C(lit.) |
| Density | 1.2 |
| vapor pressure | E-isomer 3700 Pa,
Z-isomer 3500 Pa at 25 °C |
| refractive index | n |
| Flash point: | 78 °F |
| storage temp. | 2-8°C |
| solubility | soluble in Chloroform, Methanol |
| form | neat |
| color | Colorless to Light yellow to Light orange |
| Water Solubility | <0.01 g/100 mL at 16.5 ºC |
| Merck | 14,3075 |
| BRN | 1719556 |
| Exposure limits | ACGIH: TWA 1 ppm (Skin) NIOSH: TWA 1 ppm(5 mg/m3) |
| CAS DataBase Reference | 542-75-6(CAS DataBase Reference) |
| IARC | 2B (Vol. 41, Sup 7, 71) 1999 |
| NIST Chemistry Reference | 1-Propene, 1,3-dichloro-(542-75-6) |
| EPA Substance Registry System | 1,3-Dichloropropene (542-75-6) |
Safety information for 1,3-Dichloropropene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for 1,3-Dichloropropene
| InChIKey | UOORRWUZONOOLO-OWOJBTEDSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

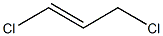
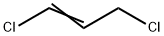

You may like
-
 1,3-Dichloropropene (cis- and trans- mixture) CAS 542-75-6View Details
1,3-Dichloropropene (cis- and trans- mixture) CAS 542-75-6View Details
542-75-6 -
 1,3-Dichloropropene CAS 542-75-6View Details
1,3-Dichloropropene CAS 542-75-6View Details
542-75-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1