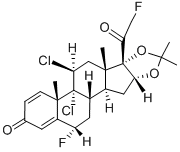
Tralonide
- CAS NO.:21365-49-1
- Empirical Formula: C24H28Cl2F2O4
- Molecular Weight: 489.38
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
What is Tralonide?
Originator
Tralonide ,Tianjin TianMao Technology Development Corp., Ltd.
Manufacturing Process
90 g of 6α-fluoro-16α,17α-isopropylidenedioxy-21-acetoxypregna-1,4,9(11)-
triene-3,20-dione are suspended in 152 ml of methanol to which has been
added 475 mg of sodium in 38 ml of methanol. This reaction mixture is stirred
for 45 min at from 20 to 25°C and then neutralized with acetic acid. After
evaporation to dryness, the residue is dissolved in methylene chloride and this
solution is washed with water, dried over sodium sulfate and concentrated.
Addition of methanol to the concentrate followed by further concentration
yields a slurry which is filtered. The solid thus collected is washed with cold
methanol and dried to yield 6α-fluoro-16α,17α-isopropylidenedioxypregna-
1,4,9(11)-trien-21-ol-3,20-dione, M.P. 245°C (dec.), [α]D = +24°.
To a cooled solution of 3.4 g of 6α-fluoro-16α,17α-isopropylidenedioxypregna-
1,4,9(11)-trien-21-ol-3,20-dione in 20 ml of 9:1 chloroform:pyridine is added
in small portions 1.4 g of tosyl chloride. The reaction mixture is allowed to
stand for 14 hours at 0°C and is then washed with dilute hydrochloric acid,
water and sodium bicarbonate solution. The chloroform is removed by
evaporation under reduced pressure and the residue is dissolved in acetone.
This acetone solution is added to a refluxing suspension of 10 g of potassium
fluoride in 50 ml of dimethylformamide. After refluxing for 5 hours, the
mixture is cooled and poured into water. The solid which forms is collected by
filtration, dried and recrystallized from acetone and hexane to yield 6α,21-
difluoro-16α,17α-isopropylidenedioxypregna-1,4,9 (11)-triene-3,20-dione, M.P.
267°C (dec.), [α]D = +9°.
5 g of 6α,21-difluoro-16α,17α-isopropylidenedioxypregna-1,4,9(11)-triene-
3,20-dione are dissolved in 50 ml of chloroform containing 5 ml of pyridine.
The mixture is held at 0°C for 15 min while a stream of chlorine is bubbled
through. The mixture is then poured into a 10% aqueous sulfuric acid solution
and the organic layer separated. This layer is washed with 5% aqueous
sodium bicarbonate and water to neutrality, dried over sodium sulfate and
evaporated to dryness to yield 6α,21-difluoro-9α,11β-dichloro-16α,17α-
isopropylidenedioxypregna-1,4-diene-3,20-dione, M.P. 245°C (dec.), [α]D =
+133°.
Therapeutic Function
Glucocorticoid
Properties of Tralonide
| Melting point: | 245 °C (decomp) |
| Boiling point: | 573.2±50.0 °C(Predicted) |
| Density | 1.36±0.1 g/cm3(Predicted) |
Safety information for Tralonide
Computed Descriptors for Tralonide
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4