TRALOMETHRIN
Synonym(s):(S)-α-Cyano-3-phenoxybenzyl (1R,3S)-2,2-dimethyl-3-[(RS)-1,2,2,2-tetrabromoethyl]cyclopropanecarboxylate
- CAS NO.:66841-25-6
- Empirical Formula: C22H19Br4NO3
- Molecular Weight: 665.01
- MDL number: MFCD00210380
- EINECS: 266-493-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-23 17:25:30
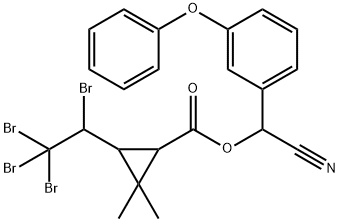
What is TRALOMETHRIN?
Description
Orange-to-yellow resinous solid (technical grade >39%)
The Uses of TRALOMETHRIN
Tralomethrin can be used as insecticide.
The Uses of TRALOMETHRIN
Tralomethrin controls a range of insects, especially Lepidoptera, in cereals, coffee, cotton, fruit, maize, oilseed rape, rice, tobacco and vegetables. It is also effective for wood protection, household use, in public health, in stored grains and for ectoparasite control on animals.
Definition
ChEBI: A carboxylic ester resulting from the formal condensation between (1R)-cis-2,2-dimethyl-3-(1,2,2,2-tetrabromoethyl)cyclopropanecarboxylic acid and the alcoholic hydroxy group of (2S)-hydroxy(3-phenoxypheny )acetonitrile.
Agricultural Uses
Insecticide: Not approved for use in EU countries. Registered for use in the U.S.
Trade name
DETHMOR®; HAG-107®; RU-25472®; RU-25474®; SCOUT®; SCOUT® X-TRA Gel insecticide
Metabolic pathway
Tralomethrin probably owes most of its action to rapid conversion by several mechanisms to deltamethrin. Information on its photodegradation, degradation in soil, and its metabolism in plants, insects and animals has been published and supports this conclusion.
Degradation
Tralomethrin is stable as a solid but is labile to base. Acidic media reduce the tendency to hydrolysis and epimerisation. It was readily debrominated when solutions or thin films were irradiated at 360 nm or by sunlight. Homolytic fission of a C-Br bond initiates this reaction, which affords deltamethrin (2). Some epimerisation to trans-tralomethrin and thence trans-deltamethrin also occurred. Dehydrobromination also affords deltamethrin and the tribromo derivative (8). Ester cleavage to the acid (3) and to the cyanohydrin (5) and thence to 3PBAl (6) and 3PBA (7) is important in the solid phase but not in solution (Ruzo and Casida, 1981). The products are shown in Scheme 1.
Toxicity evaluation
Acute oral LD50 for rats: 99-3,000 mg/kg depending on the carrier used
Properties of TRALOMETHRIN
| Melting point: | 143℃ |
| Boiling point: | 185~190℃ (0.1mmHg) |
| Density | 1.70 g/cm3 (20℃) |
| vapor pressure | 4.8×10-9 Pa (25 °C) |
| refractive index | 1.6300 (estimate) |
| storage temp. | 0-6°C |
| form | neat |
| Water Solubility | 0.08 mg l-1 |
| EPA Substance Registry System | Tralomethrin (66841-25-6) |
Safety information for TRALOMETHRIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for TRALOMETHRIN
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Tralomethrin CAS 66841-25-6View Details
Tralomethrin CAS 66841-25-6View Details
66841-25-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
