Tolcapone
Synonym(s):3,4-Dihydroxy-4′-methyl-5-nitrobenzophenone;Ro 40-7592
- CAS NO.:134308-13-7
- Empirical Formula: C14H11NO5
- Molecular Weight: 273.24
- MDL number: MFCD00866569
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
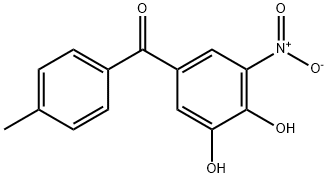
What is Tolcapone?
Absorption
Rapidly absorbed (absolute bioavailability is about 65%)
Toxicity
LD50 = 1600 mg/kg (Orally in rats)
Description
Tolcapone (134308-13-7) is a catechol O-methyltransferase inhibitor (COMT), inhibiting both brain and peripheral enzymes.1?Potent inhibitor of alpha-synuclein and beta-amyloid oligomerization and fibrillogenesis protecting against extracellular toxicity.2?Binds to transthyretin (TTR) with high affinity (21 to 58 nM) and inhibits TTR aggregation in human plasma and prevents TTR-induced cytotoxicity?in vitro. Stabilizes TTR in mice and humans?in vivo.3?Inhibits O-methylation of exogenous polyphenols such as EGCG.4?Cell permeable. Orally bioavailable.
Chemical properties
Yellow Solid
Originator
Tasmar,Roche
The Uses of Tolcapone
Tolcapone may be used in COMT-mediated cell signaling studies.
Tolcapone has been used in methyltransferase assay in human embryonic kidney 293 cells.
The Uses of Tolcapone
Orally active inhibitor of central and peripheral catechol-O-methyltransferase (COMT). Antiparkinsonian
Indications
Used as an adjunct to levodopa/carbidopa therapy for the symptomatic treatment of Parkinson's Disease. This drug is generally reserved for patients with parkinsonian syndrome receiving levodopa/carbidopa who are experiencing symptom fluctuations and are not responding adequately to or are not candidates for other adjunctive therapies.
Background
Tolcapone is a drug that inhibits the enzyme catechol-O-methyl transferase (COMT). It is used in the treatment of Parkinson's disease as an adjunct to levodopa/carbidopa medication. It is a yellow, odorless, non-hygroscopic, crystalline compound. Tolcapone is associated with a risk of hepatotoxicity.
What are the applications of Application
Tolcapone is an inhibitor of peripheral and central catechol-O-methyltransferase
Preparation
preparation by demethylation of 4-hydroxy-3-methoxy-4′-methyl-5-nitrobenzophenone with hydrobromic acid in refluxing aque-ous acetic acid.
Definition
ChEBI: Tolcapone is benzophenone substituted on one of the phenyl rings at C-3 and C-4 by hydroxy groups and at C-5 by a nitro group, and on the other phenyl ring by a methyl group at C-4. It is an inhibitor of catechol O-methyltransferase. It has a role as an EC 2.1.1.6 (catechol O-methyltransferase) inhibitor and an antiparkinson drug. It is a member of benzophenones, a member of 2-nitrophenols and a member of catechols.
Manufacturing Process
Condensation of 4-benzyloxy-3-methoxybenzaldehyde with 4-lithium-toluene (prepared from 4-bromotoluene and butyl lithium) leads to the corresponding benzhydrole. Oxidation of the new formed hydroxyl in benzhydrole gives the 4-benzyloxy-3-methoxyphenyl)-p-tolylmethanone. Treatment of this compound with hydrogen bromide selectively removes the benzyl ether that is additionally activated by the transannular carbonyl group. The intermediate is then nitrated under standart conditions to give the (4-hydroxy-3-methoxy-5- nitrophenyl)-p-tolylmethanone. A second treatment of the last compound with hydrogen bromide cleaves the ether group, which is now activated by the adjacent nitro group. This last step affords the (3,4-dihydroxy-5-nitrophenyl)(4-methylphenyl)methanone.
Therapeutic Function
Antiparkinsonian
General Description
Tolcapone, 3,4-dihydroxy-4'-methyl-5-nitrobenzophenone (Tasmar), is a yellow, odorless, nonhygroscopic,crystalline compound (pKa=4.78). Tolcapone israpidly absorbed after oral administration. Tolcapone ishighly bound to plasma albumin (>98%), and its distributionis therefore restricted. Tolcapone has low first-pass metabolism.It is almost completely metabolized in the liver before excretion, and 60% is excreted by the kidney. The majormetabolite of tolcapone is an inactive glucuronide conjugate.COMT inhibitors increase chronotropic and arrhythmogeniceffects of epinephrine.Tolcapone is indicated as an adjunctto levodopa/carbidopa for the management of signs andsymptoms of PD.
Biochem/physiol Actions
Tolcapone is an orally active catechol-O-methyltransferase (COMT) inhibitor. It inhibits both central and peripheral COMT. Tolcapone crosses the blood-brain barrier, and has been used for L-DOPA adjunct therapy in the treatment of Parkinson′s Disease.
Pharmacokinetics
Tolcapone is a potent, selective, and reversible inhibitor of catechol-O-methyltransferase (COMT). In humans, COMT is distributed throughout various organs. COMT catalyzes the transfer of the methyl group of S-adenosyl-L-methionine to the phenolic group of substrates that contain a catechol structure. Physiological substrates of COMT include dopa, catecholamines (dopamine, norepinephrine, epinephrine) and their hydroxylated metabolites. The function of COMT is the elimination of biologically active catechols and some other hydroxylated metabolites. COMT is responsible for the elimination of biologically active catechols and some other hydroxylated metabolites. In the presence of a decarboxylase inhibitor, COMT becomes the major metabolizing enzyme for levodopa catalyzing it to 3-methoxy-4-hydroxy-L-phenylalanine (3-OMD) in the brain and periphery. When tolcapone is given in conjunction with levodopa and an aromatic amino acid decarboxylase inhibitor, such as carbidopa, plasma levels of levodopa are more sustained than after administration of levodopa and an aromatic amino acid decarboxylase inhibitor alone. It is believed that these sustained plasma levels of levodopa result in more constant dopaminergic stimulation in the brain, leading to greater effects on the signs and symptoms of Parkinson's disease in patients as well as increased levodopa adverse effects, sometimes requiring a decrease in the dose of levodopa.
Clinical Use
Catechol-o-methyltransferase inhibitor:
Treatment of Parkinson’s disease
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: avoid with MAOIs.
Metabolism
The main metabolic pathway of tolcapone is glucuronidation
Metabolism
Extensively metabolised, mainly by conjugation to
the inactive glucuronide, but methylation by catecholO-methyltransferase to 3-O-methyltolcapone and
metabolism by cytochrome P450 isoenzymes CYP3A4
and CYP2A6 also occurs.
Approximately 60
% of a dose is excreted in the urine with
the remainder appearing in the faeces.
storage
Store at +4°C
References
1) Manisto?et al.?(1992),?Different in vivo properties of three new inhibitors of catechol O-methyltransferase in the rat; Br, J. Pharmacol.,?105?569 2) Giovanni?et al.?(2010),?Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of alpha-synuclein and beta-amyloid and protect against amyloid-induced toxicity;?J. Biol. Chem.,?285?14941 3) Sant’Anna?et al.?(2016),?Repositioning tolcapone as a potent inhibitor of transthyretin amyloidogenesis and associated cellular toxicity; Nat. Commun.,?7?10787 4) Forester and Lambert (2015),?The catechol-O-methyltransferase inhibitor, tolcapone, increases the bioavailability of unmethylated (-)-epigallocatechin-3-gallate in mice; Funct. Foods,?17?183
Properties of Tolcapone
| Melting point: | 126-1280C |
| Boiling point: | 485.6±45.0 °C(Predicted) |
| Density | 1.419±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥15mg/mL |
| pka | 4.78±0.38(Predicted) |
| form | powder |
| color | yellow |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. |
| CAS DataBase Reference | 134308-13-7(CAS DataBase Reference) |
Safety information for Tolcapone
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Tolcapone
Tolcapone manufacturer
Cohance Lifesciences (Previously RA Chem Pharma Ltd)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 134308-13-7 Tolcapone 98%View Details
134308-13-7 Tolcapone 98%View Details
134308-13-7 -
 134308-13-7 98%View Details
134308-13-7 98%View Details
134308-13-7 -
 Tolcapone CAS 134308-13-7View Details
Tolcapone CAS 134308-13-7View Details
134308-13-7 -
 Tolcapone CAS 134308-13-7View Details
Tolcapone CAS 134308-13-7View Details
134308-13-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1