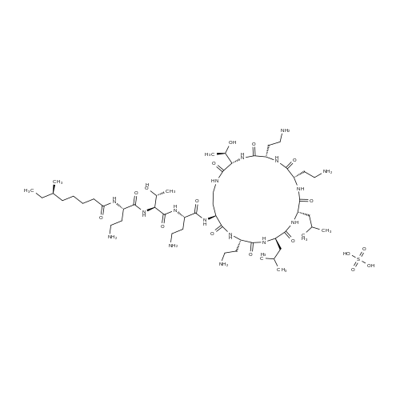
Tobramycin sulfate
- CAS NO.:49842-07-1
- Empirical Formula: C18H39N5O13S
- Molecular Weight: 565.59
- MDL number: MFCD00133864
- EINECS: 256-499-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
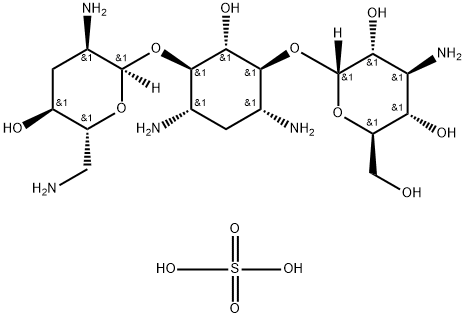
What is Tobramycin sulfate?
Originator
Distobram,Lilly,Portugal
The Uses of Tobramycin sulfate
Tobramycin sulfate is used as an antibiotic which is used to treat various types of bacterial infections, particularly Gram-negative infections. It is especially effective against species of Pseudomonas. It is used to inhibit bacterial protein synthesis at the level of 30S (16S rRNA) and 70S ribosomal complex assembly. It is used to treat pseudomonas aeruginosa lung infections and is used in combination with other antibiotics to treat urinary tract infections, gynecologic infections, peritonitis, endocarditis, pneumonia, sepsis, respiratory infections, osteomyelitis and other soft-tissue infections. It is a potential therapy for sinus infections1. Product T1783 has been used to study antibiotic resistance2.
The Uses of Tobramycin sulfate
Nebcin (Lilly).
What are the applications of Application
Tobramycin sulphate is an aminoglycoside antibiotic with an efficacy against gram-bacteria
Manufacturing Process
Two thousand parts by volume of an aqueous culture medium (pH 7.2) comprising 0.5% of glycerol, 0.5% of polypeptone, 0.5% of yeast extract and 0.3% of meat extract is inoculated with Escherichia coli R11 (IFO-13560). The medium is incubated at 37°C under aeration for 18 h. The culture broth is subjected to centrifuge to recover 4.4 parts of wet cells. The cells are suspended into 17.6 parts by volume of 0.05 M phosphate buffer (pH 7.0). The suspension is subjected to ultrasonic oscillation (Kaijo Denki Co., Ltd.; TA-4201, 4280-type, 2A) to disintegrate the cells, followed by removing the debris (insoluble materials) by centrifugation, whereby 17 parts by volume of crude enzyme solution is obtained. To 17 parts by volume of the crude enzyme solution are added 5 parts of kanamycin B, 50 parts by volume of 0.5 M phosphate buffer (pH 7.0), 100 parts by volume of 1 M adenosine triphosphate solution, 50 parts by volume of 0.1 M magnesium acetate solution and 50 parts by volume of 0.1 M 2- mercaptoethanol, which is filled up to 500 parts by volume with distilled water. The mixture is subjected to enzymic reaction at 37°C for 20 h. The reaction mixture is heated at 80°C for 5 min to cease the reaction, followed by centrifugation. The supernatant is run onto a column of 100 parts by volume of cation-exchange resin [Amberlite IRC-50, NH4 +-form]. The column is washed with water, and then eluted with 1 N-aqueous ammonia to give fractions which contain kanamycin B-3'-phosphate. The fractions are collected and concentrated under reduced pressure, and then the concentrate is run onto a column of 100 parts by volume of cation-exchange resin [carboxy-methyl Sephadex C-25, NH4 +-form]. The column is washed with water, and eluted with 0.2 N-aqueous ammonia to give fractions which contain kanamycin B-3'-phosphate. The fractions are collected, concentrated and lyophilized, whereby 4.5 parts of kanamycin B-3'-phosphate. A solution of one part of kanamycin B-3'-phosphate, 10 parts by volume of bis(trimethylsilyl)acetamide, 2 parts by volume of trimethylchlorosilane and 0.4 part of triphenylphosphine is heated at 115°C for 30 h. After cooling, the reaction mixture is concentrated under reduced pressure, and to the concentrate is added 100 parts by volume of methanol and 50 parts by volume of water, and then the mixture is stirred for 1 h. Methanol is removed by distillation, and ethyl acetate-soluble portion is removed. The water layer is run onto a column of 60 parts by volume of cation-exchange resin [Amberlite CG-50, NH4 +-form]. The column is washed with 200 parts by volume of water, and fractionated by linear gradient method with 600 parts by volume of water and 600 parts by volume of 0.5 N-aqueous ammonia, each fraction being 10 parts by weight. Upon concentration of some fractions 0.61 part of 2',3'- epimino-2'-deamino-3'-deoxykanamycin B is obtained. In 40 parts by volume of water is dissolved 0.6 part of 2',3'-epimino-2'- deamino-3'-deoxykanamycin B, and in the presence of 9 parts by volume of Raney nickel the mixture is stirred while introducing hydrogen gas at a pressure of 100 kg/cm2 at 60°C for 6 h. After the reaction Raney nickel is separated by filtration. The Raney nickel is washed well with 300 parts by volume of 1 N-aqueous ammonia and the washing is added to the filtrate. The whole is concentrated to about 100 parts by volume. The precipitated insolubles are removed by filtration, and the pH of the supernatant is adjusted to about 5.0 with hydrochloric acid. The mixture is run onto a column of 50 ml of cation-exchange resin [Amberlite CG-50, NH4 +-form]. The column is washed with 150 parts by volume of water, and fractionated by linear gradient method with 1400 parts by volume of water and 1400 parts by volume of 0.3 N-aqueous ammonia, each fraction being 14 parts by weight. From No. 146 to 162 fractions 0.30 part of 3'-deoxykanamycin B (Tobramycin) is obtained. water. The mixture is subjected to enzymic reaction at 37°C for 20 h. The reaction mixture is heated at 80C for 5 min to cease the reaction, followed by centrifugation. The supernatant is run onto a column of 100 parts by volume of cation-exchange resin [Amberlite IRC-50, NH4 +-form]. The column is washed with water, and then eluted with 1 N-aqueous ammonia to give fractions which contain kanamycin B-3'-phosphate. The fractions are collected and concentrated under reduced pressure, and then the concentrate is run onto a column of 100 parts by volume of cation-exchange resin [carboxy-methyl Sephadex C-25, NH4 +-form]. The column is washed with water, and eluted with 0.2 N-aqueous ammonia to give fractions which contain kanamycin B-3'-phosphate. The fractions are collected, concentrated and lyophilized, whereby 4.5 parts of kanamycin B-3'-phosphate. A solution of one part of kanamycin B-3'-phosphate, 10 parts by volume of bis(trimethylsilyl)acetamide, 2 parts by volume of trimethylchlorosilane and 0.4 part of triphenylphosphine is heated at 115°C for 30 h. After cooling, the reaction mixture is concentrated under reduced pressure, and to the concentrate is added 100 parts by volume of methanol and 50 parts by volume of water, and then the mixture is stirred for 1 h. Methanol is removed by distillation, and ethyl acetate-soluble portion is removed. The water layer is run onto a column of 60 parts by volume of cation-exchange resin [Amberlite CG-50, NH4 +-form]. The column is washed with 200 parts by volume of water, and fractionated by linear gradient method with 600 parts by volume of water and 600 parts by volume of 0.5 N-aqueous ammonia, each fraction being 10 parts by weight. Upon concentration of some fractions 0.61 part of 2',3'- epimino-2'-deamino-3'-deoxykanamycin B is obtained. In 40 parts by volume of water is dissolved 0.6 part of 2',3'-epimino-2'- deamino-3'-deoxykanamycin B, and in the presence of 9 parts by volume of Raney nickel the mixture is stirred while introducing hydrogen gas at a pressure of 100 kg/cm2 at 60°C for 6 h. After the reaction Raney nickel is separated by filtration. The Raney nickel is washed well with 300 parts by volume of 1 N-aqueous ammonia and the washing is added to the filtrate. The whole is concentrated to about 100 parts by volume. The precipitated insolubles are removed by filtration, and the pH of the supernatant is adjusted to about 5.0 with hydrochloric acid. The mixture is run onto a column of 50 ml of cation-exchange resin [Amberlite CG-50, NH4 +-form]. The column is washed with 150 parts by volume of water, and fractionated by linear gradient method with 1400 parts by volume of water and 1400 parts by volume of 0.3 N-aqueous ammonia, each fraction being 14 parts by weight. From No. 146 to 162 fractions 0.30 part of 3'-deoxykanamycin B is obtained. The aqueous solution containing 3'-deoxykanamycin B in free base form by addition of concentrated sulfuric acid give the 3'-deoxykanamycin B sulfate (tobramycin sulfate). The solution of this compound is decolorized by stirring of Darco G-60, filtered and purified by column chromatography.
Therapeutic Function
Antibiotic
Clinical Use
Introduced in 1976, tobramycin sulfate (Nebcin) is the mostactive of the chemically related aminoglycosides called nebramycinsobtained from a strain of Streptomyces tenebrarius Five members of the nebramycin complex have beenidentified chemically.
Factors 4 and 4' are 6"-O-carbamoylkanamycin B andkanamycin B, respectively; factors 5' and 6 are 6"-O-carbamoyltobramycinand tobramycin; and factor 2 isapramycin, a tetracyclic aminoglycoside with an unusual bicycliccentral ring structure. Kanamycin B and tobramycinprobably do not occur in fermentation broths per se but areformed by hydrolysis of the 6-O"-carbamoyl derivatives inthe isolation procedure.
The most important property of tobramycin is its activityagainst most strains of P. aeruginosa, exceeding that of gentamicinby twofold to fourfold. Some gentamicin-resistantstrains of this troublesome organism are sensitive to tobramycin,but others are resistant to both antibiotics. OtherGram-negative bacilli and staphylococci are generally moresensitive to gentamicin. Tobramycin more closely resembleskanamycin B in structure (it is 3'-deoxykanamycin B).
Properties of Tobramycin sulfate
| storage temp. | 2-8°C |
| solubility | Methanol (Slightly), Water (Sparingly) |
| form | Powder |
| color | White to off-white |
| Water Solubility | Soluble in water (50 mg/ml). |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 49842-07-1 |
| EPA Substance Registry System | Tobramycin sulfate (49842-07-1) |
Safety information for Tobramycin sulfate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P330:Rinse mouth. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for Tobramycin sulfate
Tobramycin sulfate manufacturer
Horster Biotek Pvt Ltd
Ralington Pharma
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Tobramycin sulfate 99%View Details
Tobramycin sulfate 99%View Details -
 79645-27-5 / 49842-07-1 98%View Details
79645-27-5 / 49842-07-1 98%View Details
79645-27-5 / 49842-07-1 -
 Tobramycin sulfate CAS 49842-07-1View Details
Tobramycin sulfate CAS 49842-07-1View Details
49842-07-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1