TLCK
Synonym(s):(3S)-1-Chloro-3-tosylamido-7-amino-2-heptanone hydrochloride;(3S)-7-Amino-1-chloro-3-tosylamino-2-heptanone hydrochloride;TLCK;Tosyl-L -lysyl-chloromethane hydrochloride
- CAS NO.:4272-74-6
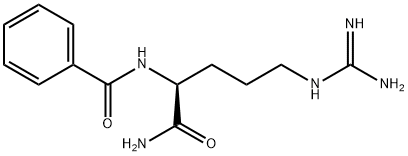
- Empirical Formula: C14H21ClN2O3S.ClH
- Molecular Weight: 369.31
- MDL number: MFCD00065395
- EINECS: 224-266-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
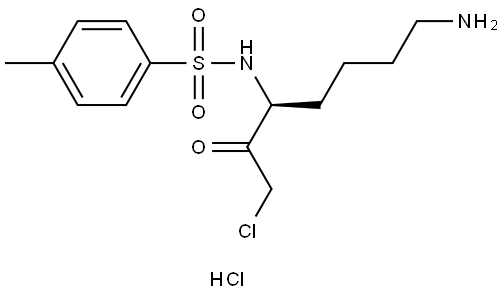
What is TLCK?
Chemical properties
white to light beige powder
The Uses of TLCK
Tosyllysine chloromethylketone (TLCK) is an active site-directed agent that inhibits serine proteinases with trypsin-like activity. TLCK may also act non-selectively with thiol groups and thereby inhibit cysteine proteinases and other enzymes. To prevent proteolytic degradation, TLCK may be used in protein purification protocols. TLCK selectively inactiviates clostripain obtained from C. histolyticum.
The Uses of TLCK
Nα-Tosyl-L-lysine chloromethyl ketone hydrochloride has been used:
- in chymotrypsin purification to prevent binding of trypsins to the affinity support by inhibiting them in the crude extract
- as a protease inhibitor in tissue and cell homogenization
- as a trypsin inhibitor to determine the specific protease activity levels
Biological Activity
tosyllysine chloromethyl ketone (hydrochloride) is a protease inhibitor [1][2][3].l-1-chloro-3-[4-tosylamido]-7-amino-2-heptanone-hcl (tlck) is a protease inhibitor. tlck is an active site-directed agent that inhibits serine proteinases with trypsin-like activity. tlck also interacts non-selectively with thiol groups and thereby inhibits cysteine proteinases and other enzymes. in lps-activated rat alveolar macrophages, tlck at 1-100 μm inhibited nox- accumulation and inducible inos expression in a concentration-dependent way [1]. to prevent proteolytic degradation, tlck may be used in protein purification protocols [2]. tlck significantly increased the cytotoxic activity of c. histolyticum supernatant towards human epithelial hela cells probably by hindering natural defence mechanisms of cells. 30 min incubation with bacterial supernatant increased toxicity in both concentrations (200 and 1000 μm) from 18 ± 3% to 39 ± 3% and 57 ± 8%, respectively. tlck also blocked clostripain enzymatic activity obtained from c. histolyticum. so tlck might be used to treat diseases complicated by concurrent c. histolyticum infection [3].
Biochem/physiol Actions
Nα-Tosyl-L-lysine chloromethyl ketone hydrochloride (TLCK) blocks the lipopolysaccharide (LPS)- or cytokine-induced activation of nuclear factor κB (NF-κB), which, in turn, blocks the induction of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) transcription. Blocks activation of pp70s6k by all mitogens. Blocks apoptosis in cell lines by inhibiting the processing of caspases in some cell lines and to some stimuli.
Purification Methods
The hydrochloride slowly crystallises from a concentrated solution in absolute EtOH, thinned with EtOH/Et2O for collection and dried in vacuo. It is a suicide enzyme inhibitor of serine proteases, e.g. trypsin and clostripain. [Matsuda et al. Chem Pharm Bull Jpn 30 2512 1982, Shaw et al. Biochemistry 4 2219 1965].
References
[1]. griscavage jm, wilk s, ignarro lj. serine and cysteine proteinase inhibitors prevent nitric oxide production by activated macrophages by interfering with transcription of the inducible no synthase gene. biochem biophys res commun. 1995 oct 13;215(2):721-9.
[2]. urban mk, franklin sg, zweidler a. isolation and characterization of the histone variants in chicken erythrocytes. biochemistry. 1979 sep 4;18(18):3952-60.
[3]. józwiak, j.,komar, a.,jankowska, e., et al. determination of the cytotoxic effect of clostridiumhistolyticum culture supernatant on hela cells in the presence of protease inhibitors. fems immunology & medical microbiology 45(2):137-42 (2005).
Properties of TLCK
| Melting point: | ~165 °C (dec.) |
| storage temp. | -20°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| color | white to pink |
| optical activity | [α]20/D 7.8±0.5°, c = 2% in H2O |
| BRN | 7106867 |
| CAS DataBase Reference | 4272-74-6 |
Safety information for TLCK
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for TLCK
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran




You may like
-
 Nα-Tosyl-L-lysine chloromethyl ketone hydrochloride CAS 4272-74-6View Details
Nα-Tosyl-L-lysine chloromethyl ketone hydrochloride CAS 4272-74-6View Details
4272-74-6 -
 (9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
(9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
82911-69-1 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 1421517-99-8 99.0%View Details
1421517-99-8 99.0%View Details
1421517-99-8 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 2-methyl-5-nitrophenol 98.0%View Details
2-methyl-5-nitrophenol 98.0%View Details
5428-54-6 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3