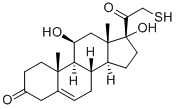
Tixocortol
- CAS NO.:61951-99-3
- Empirical Formula: C21H30O4S
- Molecular Weight: 378.53
- MDL number: MFCD00866066
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-29 08:28:14
What is Tixocortol?
Absorption
The absorption of tixocortol is the same as in other steroids including hydrocortisone. Oral administration of tixocortol presents a 10-20% bioavailability with a significantly lower plasma Cmax than cortisol. The fast metabolism, larger volume of distribution and low bioavailability donates tixocortol with the absence of systemic activity.
Toxicity
Diverse studies performed on tixocortol proved that this drug is non-toxic and non-immunosupressive. This low toxicity and abscence of immuno supression gave tixocortol the potential to be a lead for topical or local anti-inflammatory treatments. Nevertheless, toxicortol is a potent cutaneous sensitizer, causing a local allergy.
Originator
Pivalone,Jouveinal,France,1978
The Uses of Tixocortol
Tixocortol is an anti-inflammatory agent. Tixocortol is used in fluorescent chemoaffinity labeling.
Background
Tixocortol is a 21-thiol derivative of hydrocortisone classified as a class A corticosteroid. It is a synthetic steroid with topical anti-inflammatory properties without the systemic glucocorticoid and mineralocorticoid activities and toxicity.
Indications
Tixocortol is indicated for the treatment of rhinitis as a nasal suspension or aerosol. It is also used in the form of lozenges for the treatment of pharyngitis and in the form of enemas or rectal solution for the treatment of ulcerative colitis. Tixocortol can be used orally in a suspension or powder for the treatment of inflammatory conditions. It is also the substance used for the screening of contact allergies to class A steroids.
Definition
ChEBI: A steroid sulfide in which the sulfanyl group is at C-21 of a polyoxygenated derivative of pregn-4-ene.
Manufacturing Process
In a reactor of 50 liters, sodium S-thiopivalate is prepared from 100 g of S_x0002_thiopivalic acid (0.844 mol), 214 cc of solution of sodium methylate, 3.95 M
(0.844 mol) in 25 liters of anhydrous acetone.
There are then added 285 g (0.603 mol) of dihydroxy-11β,17α-iodo-21-dioxo-
3,20-pregnene-4 and the mixture is brought up to the acetone reflux for two
hours. The solvent is eliminated by distillation under vacuum until there is
obtained a syrupy residue which is poured into 10 liters of iced water. The
insoluble part is filtered and dried under vacuum.
The crude product is purified by recrystallization from ethanol; weight: 250 g;
yield: 89.5%.
Therapeutic Function
Antiinflammatory
Pharmacokinetics
Tixocortol presents the characteristic of local action which reduces significantly the side effects of systemic glucocorticoids. Reports have demonstrated that gastrointestinal administration of tixocortol generates a decrease in abdominal pain, bleeding, and frequency of stools which resulted in an amelioration in the malabsorption laboratory tests. All the effects were independent of suppression of the pituitary-adrenal axis, which was shown by the absence of significant depression of cortisol. Administration of tixocortol as a nasal spray has been shown to respect nasal drainage by the ciliary beats of the pituitary mucosa. The actions of tixocortol have no effect on leukocyte count, blood glucose level, sodium urinary excretion, and immunosupressive activity on lymphocytes.
Metabolism
Tixocortol is rapidly modified within red blood cells and it is immediately metabolized by a first-pass liver metabolism. The metabolites of tixocortol are mainly represented by the formation of sulfo- and glucurono-conjugates which are later hydrolyzed from the conjugate forming neutral steroids. The metabolic transformations are the reduction of the 3-keto and delta 4 system, reduction of the C-20 carbonyl group, oxidation of the C-11 alcohol and cleavage of the side chain at C-17. The specific metabolic pathways of the C-21 thiol ester function were its transformation into methylthio, methylsulfonyl and methylsulfonyl derivatives and reductive cleavage of the C-21-S bond leading to 21-methyl structures. None of the metabolites have affinity for glucocorticoid receptors. This and the extensive metabolism explains the exclusive local activities of tixocortol.
Safety information for Tixocortol
New Products
3-Oxocyclobutane-1-carboxylicacid 4-(Ethylaminomethyl)pyridine Ethyl 3-pyridylacetate 5-Chloro-1-(4-piperidyl)-2-benzimidazolinone Pyridin-4-ylmethanol hydrobromide Trans-methyl 4-aminocyclohexane- carboxylate HCl 1-Indanone 4-Chloro Phenyl hydrazine hydrochloride Nintedanib Ethanesulfonate (S)-2-(3,5-diMethylphenyl)pyrrolidine Ethyl 2-(4-(chlorosulfonyl)-2-methylphenoxy)acetate (2R,3S,4S,5R)-3-(3,4-difluoro-2-methoxyphenyl)-4,5-dimethyl-5-(trifluoromethyl)tetrahydrofuran-2-carboxylic acid (R)-1-phenylethan-1-amine salt 1-Bromo-2-Methoxy-3-Nitrobenzene N N N'Trimethyl ethylenediamine N Ethylmethylamine Ethyl Methanesulfonate Variamine Blue B Diazonium salt Lead II Bromide N N' DimethylEthylenediamine L-Glycine methyl ester.HCl Calcium Alphaketoglutarate* Fmoc-L-Glu(OtBu)-OH.H2O H-Ser(t-Bu)-Ser(t-Bu)-Gly-OH Fmoc-Ser(tBu)-Ser(Ψ(Me,Me)pro-OHRelated products of tetrahydrofuran
You may like
-
 2-Fluoro-6-iodobenzoic acid 111771-08-5 98+View Details
2-Fluoro-6-iodobenzoic acid 111771-08-5 98+View Details
111771-08-5 -
![3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+](https://img.chemicalbook.in//Content/image/CP5.jpg) 3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+View Details
3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+View Details
2304754-51-4 -
 2,3-Difluoro-6-methoxybenzyl Chloride 1073435-67-2 98+View Details
2,3-Difluoro-6-methoxybenzyl Chloride 1073435-67-2 98+View Details
1073435-67-2 -
 tert-butyl 2-(4-amino-6-chloropyrimidin-5-yloxy)ethylmethylcarbamate 1787294-51-2 98+View Details
tert-butyl 2-(4-amino-6-chloropyrimidin-5-yloxy)ethylmethylcarbamate 1787294-51-2 98+View Details
1787294-51-2 -
 1589503-95-6 98+View Details
1589503-95-6 98+View Details
1589503-95-6 -
 2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 98+View Details
2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 98+View Details
2477812-43-2 -
 Phenylazomalononitrile 98+View Details
Phenylazomalononitrile 98+View Details
6017-21-6 -
 KT-474 98+View Details
KT-474 98+View Details
2432994-31-3