Tin
Synonym(s):Metallic tin;Silver Matt Powder;Tin;Tin element;Tin Powder
- CAS NO.:7440-31-5
- Empirical Formula: Sn
- Molecular Weight: 118.71
- MDL number: MFCD00133862
- EINECS: 231-141-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:37

What is Tin?
Description
Tin has a long, colorful history. The extraction and use of tin began during the Bronze Age around 3000 BC when early craftsmen discovered that bronze – a noncorrosive metal that is extremely hard and strong enough to be used for spears, swords, arrows, and other especially important objects at that time – could be produced by smelting tin with copper. Tin is also the primary constituent of pewter. Long ago, people developed the belief that trace amounts of tin seemed to help prevent fatigue and depression, and that drinking out of tin cups could help combat these ailments. Tin toys, tin coated cans, and tin roofs have also enjoyed great popularity in the past.
Chemical properties
Tin is a gray to almost silver-white, ductile, malleable, lustrous metal.
Physical properties
Tin is a soft, silvery-white metal located in the carbon group, similar in appearance to freshcutaluminum. When polished, it takes on a bluish tint caused by a thin protective coatingof oxidized tin. This property makes it useful as a coating for other metals. It is malleable andductile, meaning it can be pounded, rolled, and formed into many shapes, as well as “pulled”into wires through a die.
There are two allotropes of tin. One is known as gray or alpha (α) tin, which is not verystable. The other is known as white tin or beta (β), which is the most common allotrope. Thetwo forms (allotropes) of tin are dependent on temperature and crystalline structure. Whitetin is stable at about 13.2°C. Below this temperature, it turns into the unstable gray alphaform. There is also a lesser-known third allotrope of tin called “brittle tin,” which exists above161°C. Its name is derived from its main property.
Tin’s melting point is 231.93°C, its boiling point is 2,602°C, and the density is 5.75 g/cm3for the gray allotrope (alpha) and 7.287 g/cm3 for the white allotrope (beta).
Isotopes
There are 49 isotopes of tin, 10 of which are stable and range from Sn-112to Sn-124. Taken together, all 10 stable isotopes make up the natural abundance of tinfound on Earth. The remaining 39 isotopes are radioactive and are produced artificially innuclear reactors. Their half-lives range from 190 milliseconds to 1×10+5 years.
Origin of Name
The name “tin” is thought to be related to the pre-Roman Etruscan god Tinia, and the chemical symbol (Sn) comes from stannum, the Latin word for tin.
Occurrence
Tin is the 49th most abundant element found in the Earth’s crust. Although tin is nota rare element, it accounts for about 0.001% of the Earth’s crust. It is found in deposits inMalaysia, Thailand, Indonesia, Bolivia, Congo, Nigeria, and China. Today, most tin is minedas the mineral ore cassiterite (SnO2), also known as tinstone, in Malaysia. Cassiterite is tin’smain ore. There are no significant deposits found in the United States, but small deposits arefound on the southeast coast of England. To extract tin from cassiterite, the ore is “roasted” ina furnace in the presence of carbon, thereby reducing the metal from the slag.
History
Known to the ancients. Tin is found chiefly in cassiterite (SnO2). Most of the world’s supply comes from China, Indonesia, Peru, Brazil, and Bolivia. The U.S. produces almost none, although occurrences have been found in Alaska and Colorado. Tin is obtained by reducing the ore with coal in a reverberatory furnace. Ordinary tin is composed of ten stable isotopes; thirty-six unstable isotopes and isomers are also known. Ordinary tin is a silver-white metal, is malleable, somewhat ductile, and has a highly crystalline structure. Due to the breaking of these crystals, a “tin cry” is heard when a bar is bent. The element has two allotropic forms at normal pressure. On warming, gray, or α tin, with a cubic structure, changes at 13.2°C into white, or β tin, the ordinary form of the metal. White tin has a tetragonal structure. When tin is cooled below 13.2°C, it changes slowly from white to gray. This change is affected by impurities such as aluminum and zinc, and can be prevented by small additions of antimony or bismuth. This change from the α to β form is called the tin pest. Tin–lead alloys are used to make organ pipes. There are few if any uses for gray tin. Tin takes a high polish and is used to coat other metals to prevent corrosion or other chemical action. Such tin plate over steel is used in the so-called tin can for preserving food. Alloys of tin are very important. Soft solder, type metal, fusible metal, pewter, bronze, bell metal, Babbitt metal, white metal, die casting alloy, and phosphor bronze are some of the important alloys using tin. Tin resists distilled sea and soft tap water, but is attacked by strong acids, alkalis, and acid salts. Oxygen in solution accelerates the attack. When heated in air, tin forms SnO2, which is feebly acid, forming stannate salts with basic oxides. The most important salt is the chloride (SnCl2 · H2O), which is used as a reducing agent and as a mordant in calico printing. Tin salts sprayed onto glass are used to produce electrically conductive coatings. These have been used for panel lighting and for frost-free windshields. Most window glass is now made by floating molten glass on molten tin (float glass) to produce a flat surface (Pilkington process). Of recent interest is a crystalline tin–niobium alloy that is superconductive at very low temperatures. This promises to be important in the construction of superconductive magnets that generate enormous field strengths but use practically no power. Such magnets, made of tin–niobium wire, weigh but a few pounds and produce magnetic fields that, when started with a small battery, are comparable to that of a 100 ton electromagnet operated continuously with a large power supply. The small amount of tin found in canned foods is quite harmless. The agreed limit of tin content in U.S. foods is 300 mg/kg. The trialkyl and triaryl tin compounds are used as biocides and must be handled carefully. Over the past 25 years the price of commercial tin has varied from 50¢/lb ($1.10/kg) to about $6/kg. Tin (99.99% pure) costs about $260/kg.
Characteristics
Although tin is located in group 14 as a metalloid, it retains one of the main characteristicsof metals: in reacting with other elements, it gives up electrons, forming positive ions just asdo all metals.
Tin has a relatively low melting point (about 231°C or 4,715°F), and it reacts with someacids and strong alkalis, but not with hot water. Its resistance to corrosion is the main characteristicthat makes it a useful metal.
There is an interesting historical event related to the two main allotropes of tin. At temperaturesbelow 13 degrees centigrade, “white” tin is slowly transformed into “gray” tin, whichis unstable at low temperatures, and during the brutally cold winter of 1850 in Russia, thetin buttons sewn on soldiers’ uniforms crumbled as the tin changed forms. In the 1800s, tinwas also widely used for pots, pans, drinking cups, and dinner flatware. However, at very lowtemperatures, these implements also disintegrated as their chemical structure was altered.
The Uses of Tin
One of the most important uses of tin is in the coating of thin steel sheets to make “tinplate,” which in turn is used to make what is known as the “tin can.” The tin coating is thin,inexpensive to apply, and resistant to most foods for extended periods of time. Other inertcoatings are sometimes used on the inside of the can to further protect the foods for longerperiods of time.
Tin is alloyed with many metals. It is added to lead to make low-melting alloys for firepreventionsprinkler systems and easy-melting solder.
It is used for bearings, to plate electrodes, and to make pewter, Babbitt metal, and dentalamalgams.
Tin also has been mixed with other metals for making castings for letter type used in printingpresses.
Some compounds of tin are used as fungicides and insecticides. Tin is also used for “weighting”silk, to give the fabric more body and heft.
Molten glass is poured over a pool of molten tin to produce smooth, solid, flat plate andwindow glass.
The Uses of Tin
Chiefly for tin-plating and manufacture of food, beverage and aerosol containers, soldering alloys, babbitt and type metals, manufacture of tin salts, collapsible tubes, coating for copper wire. Principle component in pewter. Alloys as dental materials (silver-tin-mercury), nuclear reactor components (tin-zirconium), aircraft components (tin-titanium), bronze (copper-tin), brass.
The Uses of Tin
Tin is used in tin plating; soldering; dental alloys; collapsible tubes; in the production of tin salts.
Definition
Metallic element of atomic number 50, group IVA of the periodic system, aw 118.69, valences of 2, 4; 10 isotopes.
Definition
A white lustrous metal of low melting
point; the fourth member of group 14 of
the periodic table. Tin itself is the first distinctly
metallic element of the group even
though it retains some amphoteric properties.
Its electronic structure has outer s2p2
electrons ([Kr]4d105s25p2). The element is
of low abundance in the Earth’s crust
(0.004%) but is widely distributed, largely
as cassiterite (SnOsub>2). The metal has been
known since early bronze age civilizations
when the ores used were relatively rich but
currently worked ores are as low as 1–2% and considerable concentration must be
carried out before roasting. The metal itself
is obtained by reduction using carbon,
SnOsub>2 + C → Sn + COsub>2
Tin is an expensive metal and several
processes are used for recovering tin from
scrap tin-plate. These may involve chlorination
(dry) to the volatile SnCl4, or electrolytic
methods using an alkaline
electrolyte:
Sn+ 4OH-→ Sn(OH)42-+ 2e-(anode)
Sn(OH)42- → Sn2+ + 4OH- (cathode)
Sn2+ + 2e → Sn (cathode)
Tin does not react directly with hydrogen
but an unstable hydride, SnH4, can be
prepared by reduction of SnCl4. The low
stability is due to the rather poor overlap of
the diffuse orbitals of the tin atom with the
small H-orbitals. Tin forms both tin(II)
oxide and tin(IV) oxide. Both are amphoteric,
dissolving in acids to give tin(II) and
tin(IV) salts, and in bases to form stannites
and stannates,
SnO + 4OH- → [SnO3]4-+2H2O
stannite (relatively unstable)
SnO2 + 4OH- → [SnO4]4–+ 2H2O
stannate
The halides, SnX2, may be prepared by
dissolving tin metal in the hydrogen halide
or by the action of heat on SnO plus the hydrogen
halide. Tin(IV) halides may be prepared
by direct reaction of halogen with
the metal. Although tin(II) halides are ionized
in solution their melting points are all
low suggesting considerable covalence in
all but the fluoride. The tin(IV) halides are
volatile and essentially covalent with slight
polarization of the bonds. Tin(II) compounds
are readily oxidized to tin(IV) compounds
and are therefore good reducing
agents for general laboratory use.
Tin has three crystalline modifications
or allotropes, α-tin or ‘gray tin’ (diamond
structure), β-tin or ‘white tin’, and γ-tin;
the latter two are metallic with close
packed structures. Tin also has several isotopes.
It is used in a large number of alloys
including Babbit metal, bell metal, Britannia
metal, bronze, gun metal, and pewter
as well as several special solders.
Symbol: Sn; m.p. 232°C; b.p. 2270°C;
r.d. 7.31 (20°C); p.n. 50; r.a.m. 118.710.
Production Methods
Tin is relatively rare, composing only about 0.0006% in the earth’s crust. The major tin ore is cassiterite, a naturally occurring tin (IV) oxide (SnO2). The other major tin-containing minerals are stannate, teallite, cylindrite, and canfieldite that are sulfides of tin.
General Description
White TIN is an almost silver-white, ductile, malleable, lustrous solid. Mp 232°C; bp: 2507°C. Density: 7.3 g cm-3. Pure white TIN becomes non-metallic powdery gray TIN if held for a sustained period at temperatures less than 13°C.
Reactivity Profile
TIN is a reducing agent. Stable in massive form in air, but oxidizes (corrodes) in air as a powder, especially in the presence of water. Dissolve slowly in dilute strong acids in the cold. Dissolves in hot aqueous KOH and other strongly basic solutions. Incompatible with acids and base. Incompatible with chlorine and turpenTINe.
Hazard
Tin, as the elemental metal, is nontoxic. Most, but not all of tin’s inorganic salts and compoundsare also nontoxic.
In contrast, almost all organic tin compounds (tin compounds composed of carbon andhydrocarbons) are very toxic and should be avoided. If they are used, special equipment andcare must be taken in handling.
(Note: When chemical formulas use the letter “R” preceding an element’s symbol, it designatessome form of organic compound—for example, R4Sn. If the letter “X” follows theelement’s symbol in a formula, it designates some form of inorganic compound—for example,SnX2. Thus, a whole series of tin compounds could be designated as R4Sn2, R2Sn, or SnX4,SnX2, and so forth.)
Health Hazard
Inorganic tin salts are irritants of the eyes and skin. No systemic effects have been reported from industrial exposure. Some inorganic tin compounds can cause skin or eye irritation because of acid or alkaline reaction produced with water. Tin tetrachloride, stannous chloride, and stannous sulfate are strong acids; sodium and potassium stannate are strong alkalies.
Flammability and Explosibility
Non flammable
Industrial uses
Hot-dip coatings can be applied to fabricatedparts made of mild and alloy steels, cast iron,and copper and copper alloys to improveappearance and corrosion resistance. Like zinc,the coatings consist of two layers — a relativelypure outer layer and an intermediate alloy layer.
An invisible surface film of stannic oxideis formed during exposure, which helps toretard, but does not completely prevent, corrosion.The coatings have good resistance to tarnishingand staining indoors, and in most rural,marine, and industrial atmospheres. They alsoresist foods. Corrosion resistance in all casescan be markedly improved by increasing thicknessand controlling porosity. Typical applicationswhere they can be used are milk cans,condenser and transformer cans, food and beveragecontainers, and various items of sanitaryequipment such as cast iron mincing machinesand grinders.
Safety Profile
An inhalation hazard. Questionable carcinogen with experimental tumorigenic data by implant route. Combustible in the form of dust when exposed to heat or by spontaneous chemical reaction with Br2, BrF3, Cl2, ClF3, Cu(NO3), K2O2, S. See also POWDERED METALS and TIN COMPOUNDS.
Potential Exposure
The most important use of tin is as a protective coating for other metals, such as in the food and beverage canning industry; in roofing tiles; silverware, coated wire; household utensils; electronic components; and pistons. Common tin alloys are phosphor bronze; light brass; gun metal; high tensile brass; manganese bronze; die-casting alloys; bearing metals; type metal; and pewter. These are used as soft solders, fillers in automobile bodies; and as coatings for hydraulic brake parts; aircraft landing gear and engine parts. Metallic tin is used in the manufacture of collapsible tubes and foil for packaging. Exposures to tin may occur in mining, smelting, and refining; and in the production and use of tin alloys and solders. Inorganic tin compounds are important industrially in the production of ceramics; porcelain, enamel, glass; and inks; in the production of fungicides; anthelmintics, insecticides; as a stabilizer it is used in polyvinyl plastics and chlorinated rubber paints; and it is used in plating baths.
Carcinogenicity
Limited animal testing with stannous chloride has not revealed evidence of carcinogenic potential. Mixed results have been observed in genotoxic assays.
Environmental Fate
Tin is a naturally occurring element in the earth’s crust with
~2–3 ppm in concentration and found in environmental
media in both organic and inorganic forms. Tin may be
released to the environment from natural and anthropogenic
sources. The most significant releases of tin are from burning of
fossil fuels and industrial production and use of tin. Tin compounds are generally only sparingly soluble in water and
are likely to partition to soils, sediments, and possibly to
aquatic organisms. Inorganic tin cannot be degraded in the
environment, but may undergo redox reaction, ligand
exchange, and precipitation reactions. Inorganic tin can be
transformed into organometallic forms through the microbial
methylation process. Degradation of organotin compounds
involves the breaking of the tin–carbon bond through UV
irradiation or biological and chemical cleavage. The speciation
of organotin compounds is pH-dependent. In sediment,
organotins are generally persistent. Photodegradation of
organotins may occur at relatively slow rates. Hydrolysis is
considered insignificant. Organotin compounds may be
significantly bioconcentrated by aquatic organisms. Tin has
been historically used in antifouling paints and coatings for
the bottom of boats, but this has been discontinued because
of its extreme toxicity to marine organisms.
A bioconcentration factor (BCF) relates the concentration
of a chemical in plants and animals to the concentration of
the chemical in the medium in which they live. It was estimated
that the BCFs of inorganic tin were 100, 1000, and
3000 for marine and freshwater plants, invertebrates, and fish.
Marine algae can bioconcentrate stannic tin by a factor of
1900. The BCF of tributyltin was estimated to be 473, but
measured BCFs were always higher. Bioconcentration factors
for bis(tributyltin)oxide with marine oysters were measured as
2300–11 400. Seven-day BCFs were derived for seven organotin
compounds for muscle, liver, kidney, and vertebra tissue
of carp. The BCFs ranged from 12 to 5012; the highest factors
were found for tributyltins. However, these factors were not
based on steady-state conditions, and may be low estimates.
No information was obtained on the food chain and biomagnification
of inorganic or organic tin.
Shipping
UN3089 Metal powders, flammable, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid.
Purification Methods
Tin powder is purified by adding it to about twice its weight of 10% aqueousNaOH and shaking vigorously for 10minutes. (This removes oxide film and stearic acid or similar material that is sometimes added for pulverisation.) It is then filtered, washed with water until the washings are no longer alkaline to litmus, rinsed with MeOH and dried in air. [Sisido et al. J Am Chem Soc 83 538 1961.]
Toxicity evaluation
All organic tin compounds, including trimethyltin, triethyltin, and tributyltin compounds, have the ability to cause damages to the structure of Vitamin B12 depletion of methyl group necessary for DNA and RNA reactions. Due to lipophilicity, organotin compounds may affect lipid bilayers by altering membrane fluidity where it is an initial site of activation. Thus, these compounds can bind to proteins and inhibit mitochondrial oxidative phosphorylation (hydrolysis of adenosine triphosphate) and brain glucose oxidation and are toxic. Very little data are available on inorganic tin.
Incompatibilities
TIN is a reducing agent. Stable in bulk form in air, but as powder it corrodes (oxidizes) in air, especially in the presence of moisture. Keep away from strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Incompatible with acids, alkalies, bases, chlorine, turpentine; reacts violently with acetic aldehyde, ammonium nitrate, ammonium perchlorate, hexachloroethane. Strong reducing agents may react violently with halogens, bromine fluoride, chlorine trifluoride, copper nitrate, disulfur dichloride, nitrosyl fluoride, potassium dioxide, sodium peroxide, sulfur, and other chemicals. May form explosive compounds with hexachloroethane, pentachloroethane, picric acid, potassium iodate, potassium peroxide, 2,4,6-trinitrobenzene-1,3,5-triol.
Properties of Tin
| Melting point: | 231.9 °C (lit.) |
| Boiling point: | 2270 °C (lit.) |
| Density | 7.310 g/mL at 25 °C (lit.) |
| vapor pressure | 1Pa at 1223.85℃ |
| Flash point: | 2270°C |
| storage temp. | no restrictions. |
| solubility | H2O: soluble |
| form | wire |
| color | Silvery-gray |
| Specific Gravity | 7.31 |
| Resistivity | 11 μΩ-cm, 20°C |
| Water Solubility | reacts slowly with cold dilute HCl, dilute HNO3, hot dilute H2SO4; readily with conc HCl, aqua regia [MER06] |
| Crystal Structure | Cubic, Alpha-Tin; Diamond Structure - Space Group Fd3m |
| Merck | 13,9523 |
| Exposure limits | ACGIH: Ceiling 2 ppm OSHA: Ceiling 5 ppm(7 mg/m3) NIOSH: IDLH 50 ppm; Ceiling 5 ppm(7 mg/m3) |
| Stability: | Stable. Incompatible with strong oxidizing agents. Highly flammable as a powder. Can, in powder form, lead to dust explosions. Moisture sensitive. |
| CAS DataBase Reference | 7440-31-5(CAS DataBase Reference) |
| EPA Substance Registry System | Tin (7440-31-5) |
Safety information for Tin
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tin
| InChIKey | OLGIDLDDXHSYFE-UHFFFAOYSA-N |
Tin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
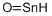


You may like
-
 Tin metal 98%View Details
Tin metal 98%View Details -
 Tin slug, 11mm (0.43 in.) dia. x 4mm (0.2 in.) thick CAS 7440-31-5View Details
Tin slug, 11mm (0.43 in.) dia. x 4mm (0.2 in.) thick CAS 7440-31-5View Details
7440-31-5 -
 Tin rod, 12.7mm (0.5 in.) dia. CAS 7440-31-5View Details
Tin rod, 12.7mm (0.5 in.) dia. CAS 7440-31-5View Details
7440-31-5 -
 Tin rod, 12.7mm (0.5 in.) dia. CAS 7440-31-5View Details
Tin rod, 12.7mm (0.5 in.) dia. CAS 7440-31-5View Details
7440-31-5 -
 Tin foil, 0.05mm (0.002 in.) thick CAS 7440-31-5View Details
Tin foil, 0.05mm (0.002 in.) thick CAS 7440-31-5View Details
7440-31-5 -
 Tin foil, 0.05mm (0.002 in.) thick CAS 7440-31-5View Details
Tin foil, 0.05mm (0.002 in.) thick CAS 7440-31-5View Details
7440-31-5 -
 Tin slug, 6.35mm (0.25 in.) dia. x 6.35mm (0.25 in.) length CAS 7440-31-5View Details
Tin slug, 6.35mm (0.25 in.) dia. x 6.35mm (0.25 in.) length CAS 7440-31-5View Details
7440-31-5 -
 Tin (metal) CASView Details
Tin (metal) CASView Details
