Tiglic acid
Synonym(s):trans-2,3-Dimethylacrylic acid;trans-2-Methyl-2-butenoic acid;Tiglic acid
- CAS NO.:80-59-1
- Empirical Formula: C5H8O2
- Molecular Weight: 100.12
- MDL number: MFCD00066864
- EINECS: 201-295-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:44
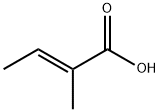
What is Tiglic acid?
Description
frarc.s-2-Methyl-2-butenoic acid has a sweet, warm, spicy odor. May be synthesized from 2-hydroxy-2-methylbutyronitrile.
Chemical properties
WHITE TO BEIGE CRYSTALLINE POWDER AND CHUNKS
Chemical properties
trans-2-Methyl-2-butenoic acid has a sweet, warm, spicy odor.
Occurrence
Reported found in celery leaves and stalk, cocoa, mango, dried bonito and Roman chamomile oil
The Uses of Tiglic acid
Tiglic acid is the stable isomer of angelic acid. Tiglic acid was found as glyceride in croton oil, as butyl ester in the oil of the Roman camomile, and as geranyl tiglate in oil of geranium. Tiglic a cid is formed during the charcoaling of maple wood.
Definition
ChEBI: A 2-methylbut-2-enoic acid having its double bond in trans-configuration.
Taste threshold values
Taste characteristics at 75 ppm: sweet, brown, fruity, with ripe and jamy nuances.
General Description
A white lustrous flaked solid with a fruity or spicy odor. About the same density as water and slightly soluble in water. May burn if heated to high temperatures. May severely irritate skin, eyes, lungs or mucous membranes.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
TIGLIC ACID is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Tiglic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Low toxicity by ingestion and skin contact. A severe skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
Synthesized from 2-hydroxy-2-methylbutyronitrile
Purification Methods
Crystallise it from water. It is steam volatile and is soluble in organic solvents. [Beilstein 2 IV 1552.]
Properties of Tiglic acid
| Melting point: | 61-64 °C(lit.) |
| Boiling point: | 95-96 °C12 mm Hg(lit.) |
| Density | 0.969 g/mL at 25 °C(lit.) |
| refractive index | nD81 1.4342 |
| FEMA | 3599 | 2-METHYL-TRANS-2-BUTENOIC ACID |
| Flash point: | 101°C |
| storage temp. | 2-8°C |
| solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| form | Crystalline Powder and Chunks |
| pka | pK (25°) 5.02 |
| color | White to beige |
| Specific Gravity | 0.969 |
| Odor | at 10.00 % in dipropylene glycol. sweet dry spicy woody caramel |
| Water Solubility | SOLUBLE IN HOT WATER |
| Merck | 14,9433 |
| JECFA Number | 1205 |
| BRN | 1236500 |
| CAS DataBase Reference | 80-59-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Butenoic acid, 2-methyl-, (E)-(80-59-1) |
| EPA Substance Registry System | 2-Butenoic acid, 2-methyl-, (2E)- (80-59-1) |
Safety information for Tiglic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tiglic acid
| InChIKey | UIERETOOQGIECD-ONEGZZNKSA-N |
Tiglic acid manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Tiglic Acid CAS 80-59-1View Details
Tiglic Acid CAS 80-59-1View Details
80-59-1 -
 Tiglic Acid extrapure CAS 80-59-1View Details
Tiglic Acid extrapure CAS 80-59-1View Details
80-59-1 -
 Tiglic Acid CAS 80-59-1View Details
Tiglic Acid CAS 80-59-1View Details
80-59-1 -
 Tiglic Acid CAS 80-59-1View Details
Tiglic Acid CAS 80-59-1View Details
80-59-1 -
 trans-2-Methyl-2-butenoic acid CAS 80-59-1View Details
trans-2-Methyl-2-butenoic acid CAS 80-59-1View Details
80-59-1 -
 Powder Tiglic Acid Chemical, For Pharmaceutical Industry, 80-59-1View Details
Powder Tiglic Acid Chemical, For Pharmaceutical Industry, 80-59-1View Details
80-59-1 -
 Tiglic AcidView Details
Tiglic AcidView Details
80-59-1 -
 Tiglic AcidView Details
Tiglic AcidView Details
80-59-1
