TIEMONIUM IODIDE
- CAS NO.:144-12-7
- Empirical Formula: C18H24INO2S
- Molecular Weight: 445.36
- MDL number: MFCD00274608
- EINECS: 205-616-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:33
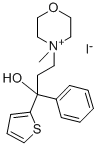
What is TIEMONIUM IODIDE?
Originator
Visceralgine,Riom,France,1963
The Uses of TIEMONIUM IODIDE
Thiemonium Iodide is found in compositions containing bicifadine for the treatment of urinary incontinence.
Definition
ChEBI: Tiemonium iodide is an aralkylamine.
Manufacturing Process
(a) N-(3-hydroxy-3-phenyl-3-α-thienyl-propyl) morpholine was first prepared:
The following quantities of reactants were mixed in a 2-liter balloon flask
having 3 tubes fitted respectively with a mercury-sealed agitator, a reflux
condenser having a calcium chloride seal, and a dropping funnel:
Magnesium turnings 27 g (1.1 g at. wt)
Bromobenzene 181 g (1.15 mol)
Anhydrous ether 500 cc
(b) To the cold Grignard solution was added a solution containing:
Thienyl-morpholinoethyl ketone 180 g (0.8 mol)
Anhydrous ether 250 cc
The ketone, preferably prepared by a Grignard reaction, was added in such a
way as to maintain the ether under constant reflux. When all of the solution
had been added, the mixture was refluxed for a further hour. The mixture was
then allowed to stand for 12 hours at ambient temperature, after which the
reaction mass was extracted with ice and ammonium chloride in known
manner.
(c) The ether solution was treated with 2 N hydrochloric acid solution and the
amino-alcohol was obtained as the hydrochloride (yield approximately 60%);
it was purified by recrystallization from methanol.
The resulting product was dissolved in water, made alkaline with dilute
NH4OHand was extracted with ether. After evaporation of the ether, the
amino-alcohol was obtained as a base.
(d) To prepare the quaternary ammonium iodide, the amino-alcohol above
was dissolved in a minimum amount of anhydrous ether and was treated with
its own weight of methyl iodide. A well-crystallized product was obtained and
was washed with anhydrous ether. (Melting point 189°C to 191°C).
Therapeutic Function
Spasmolytic, Anticholinergic
Properties of TIEMONIUM IODIDE
| Melting point: | 189-191° |
Safety information for TIEMONIUM IODIDE
Computed Descriptors for TIEMONIUM IODIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1