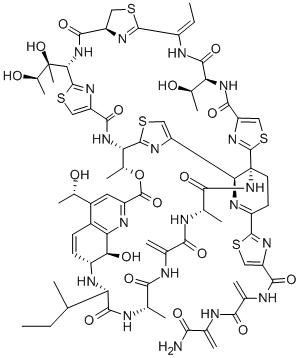
THIOSTREPTON
Synonym(s):Bryamycin;FOXM1 Inhibitor I;Thiactin;Thiostreptin A;
- CAS NO.:1393-48-2
- Empirical Formula: C72H85N19O18S5
- Molecular Weight: 1664.89
- MDL number: MFCD00135828
- EINECS: 215-734-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 15:18:15
What is THIOSTREPTON?
Description
The mammalian transcription factor forkhead box M1 (FoxM1) is induced during G1 phase, with expression continuing through S phase and mitosis. Thiostrepton is a natural peptide thiazole antibiotic that inhibits FoxM1 in mammalian cells, preventing the expression of FoxM1-regulated genes, which includes FoxM1 itself. Through this mechanism, thiostrepton prevents proliferation and induces apoptosis in human cancer cells. These effects correlate with the ability of thiostrepton to act as a proteasome inhibitor.
Chemical properties
Off-White Solid
The Uses of THIOSTREPTON
Thiostrepton is a macrocyclic antibiotic incorporating thiazoles and other atypical amino acids. Patented in 1961, thiostrepton has been used as an antibiotic and acts by binding to ribosomes to prevent the binding of the EF-G elongation factor and GTP to the 50S ribosomal subunit. Thiostrepton is an inducer of tipA, a gene that controls the bacterial transcription regulators, TipAL and TipAS, that are central regulators in multidrug resistance. Thiostrepton is closely related to siomycin, a recently discovered inhibitor of oncogenic transcription factor, FoxM1.
The Uses of THIOSTREPTON
Natural antibiotic derived from Streptomyces.
The Uses of THIOSTREPTON
beta-adrenergic agonist
The Uses of THIOSTREPTON
A protein synthesis-inhibiting antibiotic
What are the applications of Application
Thiostrepton is a cyclic peptide antibiotic, densely packed with thiazoles and other structurally intriguing moieties
Definition
ChEBI: A heterodetic cyclic peptide, in which the cyclisation step involves a formal lactonisation between the carboxy group of a quinaldic acid-based residue and a secondary alcohol. An antibiotic that inhibits bacterial protein synthesis. Also acts as an antitu or agent.
General Description
A thiazole-containing peptide antibiotic that inhibits protein synthesis by preventing binding of GTP to 50S ribosomal subunit. Inhibits the function of elongation factor G (EF-G) and the dissociation of EF-G from the ribosome. The thiostrepton-resistant gene is also commonly used as a selective marker for recombinant DNA/plasmid technologies.
Biochem/physiol Actions
Primary TargetEF-G
in vitro
thiostrepton inhibits the transcriptional activity and foxm1 expression, and induces strong apoptosis in human cancer cells of different origin that correlates with suppression of foxm1, including leukemia, neuroblastoma, liver cancer, melanoma and prostate cancer cells. thiostrepton binds foxm1 on the promoter site to inhibit transcriptional activity of foxm1 through the foxm1 autoregulation mechanism [1].
in vivo
thiostrepton suppressed tumor growth in a human breast cancer xenograft model. treatment with developed micelle-thiostrepton nanoparticles decreased xenograft tumor growth induced by the human mda-mb-231 breast and hepg2 liver cancer cell lines. these apoptosis activities in drug-treated tumors were correlated with in vivo suppression of oncogenic foxm1 [1].
References
1) Bowen et al. (2005), Interaction of thiostrepton and elongation factor-G with the ribosomal protein L11-binding domain; J. Biol. Chem., 280 2934 2) Gonzalez et al. (2007), Thiostrepton inhibition of tRNA delivery to the ribosome; RNA, 13 2091 3) Kwok et al. (2008), Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression; Mol. Cancer Ther., 7 2022
Properties of THIOSTREPTON
| Melting point: | 248-257°C (dec.) |
| alpha | D23 -98.5° (glacial acetic acid); -61° (dioxane); -20° (pyridine) |
| Density | 1.0824 (rough estimate) |
| refractive index | 1.6700 (estimate) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Soluble in DMSO or chloroform |
| form | Off-white powder |
| pka | 10.43±0.46(Predicted) |
| color | Off-white |
| Water Solubility | 0.24g/L(28 ºC) |
| Merck | 13,9440 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 week. |
Safety information for THIOSTREPTON
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for THIOSTREPTON
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Thiostrepton CAS 1393-48-2View Details
Thiostrepton CAS 1393-48-2View Details
1393-48-2 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1