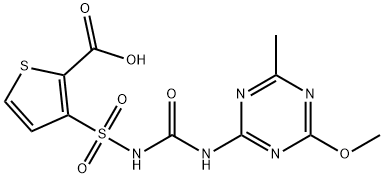
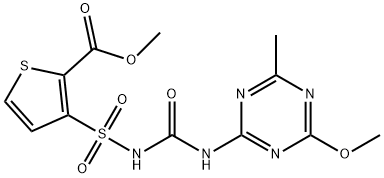
Thifensulfuron methyl
Synonym(s):Harmony
- CAS NO.:79277-27-3
- Empirical Formula: C12H13N5O6S2
- Molecular Weight: 387.39
- MDL number: MFCD00468118
- EINECS: 616-673-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Thifensulfuron methyl?
The Uses of Thifensulfuron methyl
Herbicide.
The Uses of Thifensulfuron methyl
Postemergence herbicide used to control wild garlic and many broad-leaved weeds in barley and spring wheat.
Definition
ChEBI: A methyl ester resulting from the formal condensation of the carboxy group of thifensulfuron with methanol. It is used as a post-emergence herbicide for the control of grass and broad-leaved weeds.
Agricultural Uses
Herbicide: A herbicide for postemergence broadleaf weed control in crops for food such as soybeans and cotton. Not listed for use in EU countries.
Trade name
ALLY®; BASIS® (rimsulfuron + thifensulfuron methyl); CANVAS® (thifensulfuron methyl + tribenuron methyl + metsulfuron-methyl); DPX-M6316®; EXPRESS®; HARMONY® Extra (thifensulfuron methyl + tribenuron methyl); INM-6316®; PINNACLE®; PROSPECT®; RELIANCE® SYNCHRONY®, (chlorimuron- ethyl + thifensulfuron methyl)
Environmental Fate
Chemical/Physical. May hydrolyze in aqueous solutions forming methyl alcohol and 3-(((((4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino)carbonyl)amino)sulfonyl)-2- thiophenecarboxylic acid.
Metabolic pathway
The hydrolytic degradation of thifensulfuron methyl is pH dependent and, in alkaline condition, specifically yields the corresponding free acid. Primary degradation cleaves the sulfonylurea moiety to give two typical hydrolyzed products, sulfonamide and aminotriazine analogs, derived from thifensulfuron methyl in acidic and neutral conditions. Hydrolysis of the ester linkage proceeds in mammals, plants, soils, and also chemical hydrolysis, and opening of the triazine ring occurs to yield the acetyltriuret analog identified. On the other hand, by hydrolysis, O- demethylated thifensulfuron methyl undergoes opening of the triazine ring to give the corresponding acetyltriuret analog. Under photolytic conditions, methyl 3-(4-methoxy-6-methyl-1,3,5-triazin-2- yl)aminothiophene-2-carboxylate is identified.
Properties of Thifensulfuron methyl
| Melting point: | 176°C |
| Density | 1.560±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| Water Solubility | Practically insoluble in water |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| form | Solid |
| form | neat |
| pka | pKa at 25°: 4.0 |
| color | White to Almost white |
| λmax | 280nm(Acidic aq.)(lit.) |
| Merck | 14,9316 |
| BRN | 7448062 |
| CAS DataBase Reference | 79277-27-3(CAS DataBase Reference) |
| EPA Substance Registry System | Thifensulfuron-methyl (79277-27-3) |
Safety information for Thifensulfuron methyl
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
Computed Descriptors for Thifensulfuron methyl
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 Thifensulfuron-methyl CAS 79277-27-3View Details
Thifensulfuron-methyl CAS 79277-27-3View Details
79277-27-3 -
 Thifensulfuron-methyl 95% (HPLC) CAS 79277-27-3View Details
Thifensulfuron-methyl 95% (HPLC) CAS 79277-27-3View Details
79277-27-3 -
 Thifensulfuron-methyl 95.00% CAS 79277-27-3View Details
Thifensulfuron-methyl 95.00% CAS 79277-27-3View Details
79277-27-3 -
 Thifensulfuron-methyl CAS 79277-27-3View Details
Thifensulfuron-methyl CAS 79277-27-3View Details
79277-27-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4