THALLIUM(I) NITRATE
- CAS NO.:10102-45-1
- Empirical Formula: NO3Tl
- Molecular Weight: 266.39
- MDL number: MFCD00011280
- EINECS: 233-273-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 17:11:57
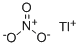
What is THALLIUM(I) NITRATE?
Chemical properties
cubic crystals
Physical properties
White crystals; exists in three allotropic modifications: a rhombohedral gamma form that transforms to trigonal beta form at 75°C, the trigonal converting to a cubic alpha form at 145°C.
Density of the salt is 5.56 g/cm3; melts at 206°C; vaporizes at 450°C with decomposition; moderately soluble in water, 9.55 g/100mL at 20°C; insoluble in alcohol.
The Uses of THALLIUM(I) NITRATE
As a reagent in analytical chemistry, especially for the determination of iodine in presence of Br and Cl; also with KClO3, HgCl and resin for green fire for signalling at sea.
Preparation
Thallium nitrate is prepared by reacting thallium metal, thallous oxide, Tl2O or thallous hydroxide, TlOH, with nitric acid followed by crystallization: Tl2O+ 2HNO3 → 2TlNO3 + H2O TlOH + HNO3 → TlNO3 + H2O.
General Description
A colorless crystalline solid melting at 206°C. Toxic by ingestion and skin absorption. Used to make other chemicals.
Air & Water Reactions
Water soluble.
Reactivity Profile
THALLIUM(I) NITRATE is an oxidizing agent. May start a fire when in contact with organic materials. Mixtures with alkyl esters may explode, owing to the formation of alkyl nitrates. Mixtures with phosphorus, tin(II) chloride, or other reducing agents may react explosively [Bretherick, 1979 p. 108-109].
Hazard
A poison. Strong oxidizing agent, fire and explosion risk. TLV: 0.1 mg(Tl)/m3. Toxic by skin absorption.
Health Hazard
Thallium is one of the more toxic elements both as an acute and a chronic poison. Effects of exposure are cumulative and onset of symptoms may be delayed 12 to 24 hours. May be fatal if inhaled, ingested or absorbed through the skin. Irritating to skin and eyes. Readily absorbed through the skin and digestive tract. Ingestion of soluble thallium compounds has caused many deaths Ingestion of sublethal quantities may cause nausea, vomiting, diarhea, abdominal pain, and bleeding from the gut accompanied or followed by drooping eyelids, crossed eyes, weakness, numbness, tingling of arms and legs, trembling, tightness and pain in the chest. Loss of hair may occur in two to three weeks. Severe intoxication may cause prostration, rapid heartbeat, convulsions, and psychosis. Some effects may be permanent.
Safety Profile
Poison by ingestion, intravenous, intraperitoneal, and subcutaneous routes. Human systemic effects by ingestion: hypermouhty, diarrhea, nausea or vomiting, and dehydration. Mutation data reported. When heated to decomposition it emits very toxic fumes of T1 and NO,. See also THALLIUM COMPOUNDS and NITRATES.
Carcinogenicity
There is inadequate carcinogenicity data. From the results of a study with thallium sulfate (383), the highest daily dose, 0.25 mg/kg, was considered a NOAEL. Using the molecular weight of TlNO3 to Tl for conversion, this NOAEL was converted to 0.26 mg TlNO3/kg per day.
Purification Methods
The nitrate crystallises from warm water (1mL/g) on cooling to 0o. POISONOUS.
Properties of THALLIUM(I) NITRATE
| Melting point: | 206 °C(lit.) |
| Boiling point: | 433 °C |
| Density | 5,55 g/cm3 |
| solubility | soluble in H2O; insoluble in ethanol |
| form | Powder or Cyrstals |
| color | White |
| Water Solubility | g/100g H2O: 3.90 (0°C), 9.55 (20°C), 414 (100°C) [LAN05]; insoluble alcohol [MER06] |
| Merck | 14,9265 |
| Stability: | Stable. Strong oxidizing agent. |
| CAS DataBase Reference | 10102-45-1(CAS DataBase Reference) |
| EPA Substance Registry System | Thallium(I) nitrate (10102-45-1) |
Safety information for THALLIUM(I) NITRATE
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for THALLIUM(I) NITRATE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION INOSITOL PANTOPRAZOLE SODIUM SESQUIHYDRATE Metformin FLUCLOXACILLIN SODIUM STERILE NIMESULIDE BP ZONISAMIDE 1,1-Dimethylethyl 4-(2-azidoacetyl)-1-piperidinecarboxylate 2-Bromo-1-(bromomethyl)-3-iodo-5-nitrobenzene 1,2-Dibromo-3-(bromomethyl)-5-nitrobenzene 2-Cyano-5-pyrimidineacetonitrile 7-Fluoro-4-cinnolinamine 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzeneRelated products of tetrahydrofuran
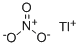
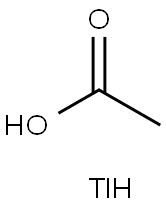
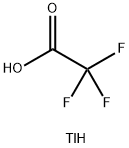

You may like
-
 Thallium(I) nitrate CAS 10102-45-1View Details
Thallium(I) nitrate CAS 10102-45-1View Details
10102-45-1 -
 Thallium(I) nitrate CAS 10102-45-1View Details
Thallium(I) nitrate CAS 10102-45-1View Details
10102-45-1 -
 1823754-06-8 98%View Details
1823754-06-8 98%View Details
1823754-06-8 -
 2-Chloro-5-(iodomethyl)pyrimidine 2268818-88-6 98%View Details
2-Chloro-5-(iodomethyl)pyrimidine 2268818-88-6 98%View Details
2268818-88-6 -
 2092793-98-9 98%View Details
2092793-98-9 98%View Details
2092793-98-9 -
 1360944-55-3 7-Methoxy-1H-indol-5-amine 98%View Details
1360944-55-3 7-Methoxy-1H-indol-5-amine 98%View Details
1360944-55-3 -
 2090480-14-9 98%View Details
2090480-14-9 98%View Details
2090480-14-9 -
![1-Methyl 2-bromo-5-[(1-carboxy-1-methylethyl)amino]benzoate 1651844-56-2 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-Methyl 2-bromo-5-[(1-carboxy-1-methylethyl)amino]benzoate 1651844-56-2 98%View Details
1-Methyl 2-bromo-5-[(1-carboxy-1-methylethyl)amino]benzoate 1651844-56-2 98%View Details
1651844-56-2