TETRAZEPAM
- CAS NO.:10379-14-3
- Empirical Formula: C16H17ClN2O
- Molecular Weight: 288.77
- MDL number: MFCD01744820
- EINECS: 233-837-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:41:45
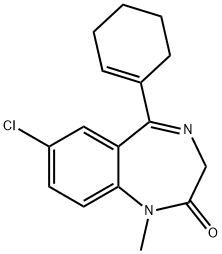
What is TETRAZEPAM?
Chemical properties
Light yellow or yellow crystalline powder.
Originator
Myolastan,Clin-Comar-Byla,France,1969
The Uses of TETRAZEPAM
Tetrazepam is a muscle relaxant (skeletal). This is a controlled substance (depressant).
Definition
ChEBI: Tetrazepam is a benzodiazepine.
Manufacturing Process
1,7-Dichloro-5-Cyclohexyl-2-Oxo-2,3-Dihydro-1H-Benzo[f]Diazepine-1,4: (a)
Process Using Sodium Hypochlorite - 40 ml of a solution of sodium
hypochlorite of 14.5 British chlorometric degrees are added to a suspension of
5.4 grams of 7-chloro-5-cyclohexyl-2-oxo-2,3-dihydro-1H-benzo[f]diazepine-
1,4 in 80 ml of methylene chloride. The mixture is stirred at room
temperature for 15 minutes; the solid dissolves rapidly. The organic layer is
decanted, washed with water, dried over anhydrous sodium sulfate and the
solvent evaporated under reduced pressure without exceeding a temperature
of 30°C. The residue is taken up in a little diisopropyl ether and the crystals
which form are dried. They are recrystallized as rapidly as possible from ethyl
acetate. Colorless crystals are obtained (3.9 grams; yield, 85%); MP = 163°C,
with decomposition.
(b) Process Using Tertiary-Butyl Hypochlorite - 1.2 grams of tertiary-butyl
hypochlorite are added to a suspension of 2.7 grams of 7-chloro-5-cyclohexyl-
2-oxo-2,3-dihydro-1H-benzo[f]diazepine-1,4 in 20 ml of methylene chloride
and the mixture is stirred and at the same time cooled in a water bath for 30
minutes. The solid dissolves in about 15 minutes. The product is evaporated
to dryness under reduced pressure at a temperature below 40°C. The residue
is taken up in diisopropyl ether and the crystals which separate are dried.
Colorless crystals are obtained (2.8 grams; yield, 98%); MP = 161° to 162°C,
with decomposition, according to US Patent 3,551,412.
7-Chloro-5-(1'-Chlorocyclohexyl)-2-Oxo-2,3-Dihydro-1H-Benzo[f]Diazepine-
1,4: A solution of 117 grams of the compound prepared above in 450 ml ethyl
acetate is heated under reflux until a precipitate begins to form. From then
onwards reflux is continued until a negative reaction is obtained when the
reaction mixture is tested with a solution of sodium iodide in acetone. The reaction mixture is left to cool and the solid which separates is dried. Colorless
crystals are obtained (76 grams), MP = 194° to 195°C, with decomposition. A
second portion (14 grams) is isolated by concentrating the mother liquor, MPk
= 194° to 195°C, with decomposition. The total yield is 77%. The melting
point is raised to 196° to 197°C by recrystallization from ethyl acetate.
7-Chloro-5-(1'-Cyclohexenyl)-2-Oxo-2,3-Dihydro-1H-Benzo[f]Diazepine-1,4:
68 grams of 7-chloro-5-(1'-chlorocyclohexyl)-2-oxo-2,3-dihydro-1Hbenzo[
f]diazepine-1,4, 34 grams of lithium carbonate and 17 grams of lithium
bromide and 340 ml of anhydrous dimethylformamide are placed in a threenecked
flask equipped with a mechanical stirrer, immersion thermometer and
a reflux condenser connected with a bubble counter.
The reaction mixture is gradually heated, with stirring, until evolution of
carbon dioxide commences (about 100°C) and the temperature is maintained
thereat until the reaction ceases. The temperature is then raised to 110°C and
held thereat for 15 minutes.
The reaction mixture is allowed to cool and the mineral salts separated and
dried. The solvent is evaporated under reduced pressure and the residue
dissolved in water. It is allowed to crystallize, dehydrated, dried and then
recrystallized from ethyl acetate. The product is yellowish crystals (47.5
grams; yield, 80%); MP = 207° to 208°C.
7-Chloro-5-(1'-Cyclohexenyl)-1-Methyl-2-Oxo-2,3-Dihydro-1HBenzo[
f]Diazepine-1,4: 9.7 grams of sodium methylate are added to a
solution of 16.5 grams of 7-chloro-5-(1'-cyclohexenyl)-2-oxo-2,3-dihydro-1Hbenzo[
f]diazepine-1,4 dissolved in 120 ml of dry dimethylformamide and the
mixture stirred for one-half hour. The reaction mixture is cooled in a water
bath and a solution of 33.8 grams of methyl iodide dissolved in 35 ml of
anhydrous dimethylformamide is then slowly added with stirring. The solution
becomes dark brown in color and a precipitate forms. It is stirred for 2 hours,
then diluted with a large volume of water and extracted with ethyl acetate.
The ethyl acetate solution is washed with water, dried over anhydrous sodium
sulfate and the solvent evaporated under reduced pressure. The residue is
crystallized from a small volume of ethyl acetate. Brownish yellow crystals are
obtained (9 grams; yield, 52%), MP = 144°C.
Therapeutic Function
Muscle relaxant
Contact allergens
Tetrazepam is a benzodiazepine compound used systemically as a myorelaxant. It may induce skin rashes such as maculo-papular eruption, Stevens-Johnson syndrome, or photosensitivity. Occupational sensitization can be observed in pharmaceutical plants. Sensitization generally does not concern other benzodiazepines.
Properties of TETRAZEPAM
| Melting point: | 144° |
| Boiling point: | 502.1±50.0 °C(Predicted) |
| Density | 1.1741 (rough estimate) |
| refractive index | 1.5800 (estimate) |
| solubility | Practically insoluble in water, freely soluble in methylene chloride, soluble in acetonitrile. |
| pka | 4.10±0.10(Predicted) |
| CAS DataBase Reference | 10379-14-3 |
Safety information for TETRAZEPAM
Computed Descriptors for TETRAZEPAM
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![7-CHLORO-2,3,4,5-TETRAHYDRO-1H-BENZO[E][1,4]DIAZEPINE](https://img.chemicalbook.in/CAS/GIF/107479-55-0.gif)



![TETRAZEPAM [CONTROLLED SUBSTANCE]](https://img.chemicalbook.in/)

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1