TETRAETHYLAMMONIUM PERCHLORATE
- CAS NO.:2567-83-1
- Empirical Formula: C8H20ClNO4
- Molecular Weight: 229.7
- MDL number: MFCD00041977
- EINECS: 219-904-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
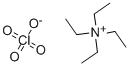
What is TETRAETHYLAMMONIUM PERCHLORATE?
Chemical properties
solid
The Uses of TETRAETHYLAMMONIUM PERCHLORATE
Tetraethylammonium perchlorate is used as supporting electrolyte in polarographic measurements.
General Description
White crystalline solid. May explode from heat, shock, or friction.
Air & Water Reactions
Water soluble.
Reactivity Profile
Oxidizing agents, such as TETRAETHYLAMMONIUM PERCHLORATE, can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds, in general, have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates. Benzene, calcium hydride, charcoal, ethanol, finely divided metals, olefins, strontium hydride, sulfur react violently with perchlorates.
Purification Methods
Crystallise the perchlorate repeatedly from water, aqueous MeOH, acetonitrile or acetone, and dry it at 70o under a vacuum for 24hours. [Cox et al. J Am Chem Soc 106 5965 1984, Liu et al. J Am Chem Soc 108 1740 1986, White & Murray J Am Chem Soc 109 2576 1987.] It has also been crystallised twice from ethyl acetate/95% EtOH (2:1) [Lexa et al. J Am Chem Soc 109 6464 1987]. [Beilstein 4 IV 332.]
Properties of TETRAETHYLAMMONIUM PERCHLORATE
| Melting point: | >300°C |
| form | Wet crystals |
| Water Solubility | Soluble in water, acetone, acetonitrile and dimethyl sulfoxide. |
| BRN | 3638093 |
| Stability: | Unstable. Heating may lead to an explosion. Contact with combustible material may cause fire. Incompatible with reducing agents, organic materials, finely powdered metals, aluminium, sulfur, benzene, calcium hydride, charcoal, alkenes, ethanol, strontium hydride, sulfuric acid. |
| CAS DataBase Reference | 2567-83-1(CAS DataBase Reference) |
| EPA Substance Registry System | Tetraethylammonium perchlorate (2567-83-1) |
Safety information for TETRAETHYLAMMONIUM PERCHLORATE
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P220:Keep/Store away from clothing/…/combustible materials. P221:Take any precaution to avoid mixing with combustibles/… P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for TETRAETHYLAMMONIUM PERCHLORATE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Tetraethylammonium perchlorate CAS 2567-83-1View Details
Tetraethylammonium perchlorate CAS 2567-83-1View Details
2567-83-1 -
 Tetraethylammonium perchlorate CAS 2567-83-1View Details
Tetraethylammonium perchlorate CAS 2567-83-1View Details
2567-83-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1