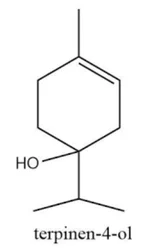
Terpinen-4-ol
Synonym(s):4-Carvomenthenol;4-Terpinenol
- CAS NO.:562-74-3
- Empirical Formula: C10H18O
- Molecular Weight: 154.25
- MDL number: MFCD00001562
- EINECS: 209-235-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18

What is Terpinen-4-ol?
Chemical properties
colourless or pale yellow liquid
Chemical properties
1-Terpinen-4-ol occurs as (+)-, (?)-, and racemic 1-terpinen-4-ol in many essential oils, for
example, from Pinus and Eucalyptus species, and in lavender oil. It is a colorless
liquid with a spicy, nutmeg-like, woody–earthy, and also lilac-like odor.
1-Terpinen-4-ol is a by-product in the synthesis of terpineol fromterpin hydrate
and occurs in commercial terpineol. Pure 1-terpinen-4-ol can be prepared from
terpinolene by photosensitized oxidation, reduction of the resulting 1-methyl-4-
isopropenyl-1-cyclohexene-4-hydroperoxide, and selective hydrogenation of the
corresponding alcohol.
It is used, for example, in artificial geranium and pepper oils and in perfumery for
creating herbaceous and lavender notes.
Occurrence
4-Carvomenthenol (dextro) has been reported present in the oil of Cupressus macrocarpa lavender, Spanish origanum, Ledum palustre, Eucalyptus australiana var. A., Thuja occidentalis, etc. The l-form is present in the oil of Eucalyptus dives and in some other essences such as Xanthoxylum rhetsa, together with the racemic form. The racemic form is found in camphor oil. Reported found in fresh apple, apricots, orange juice, peel oils of orange, lemon, grapefruit, tangerines, anise, cinnamon, ginger and nutmeg.
The Uses of Terpinen-4-ol
Shows antioxidant effects. Antiseptic.
The Uses of Terpinen-4-ol
Reference Standard in the analysis of herbal medicinal products
What are the applications of Application
rac Terpinen-4-ol is an antioxidant
Definition
ChEBI: A terpineol that is 1-menthene carrying a hydroxy substituent at position 4.
Taste threshold values
Taste characteristics at 30 ppm: sweet, citrus green with a tropical fruity character.
General Description
Produced and qualified by HWI pharma services GmbH.
Exact content by quantitative NMR can be found on the certificate.
Flammability and Explosibility
Not classified
Biochem/physiol Actions
Taste at 30 ppm
Anticancer Research
Also this molecule exhibits antitumor effects by apoptotic mechanism. Studies weredone in mice bearing A549 tumor xenografts (Quintans et al. 2013; Kiyan et al.2014).
Synthesis
One of several terpinenol isomers, depending on the position of the double bond and that of the hydroxyl group, this terpene, whose structure has been defined by Wallach, can be isolated by fractional distillation. It exists in nature as the dextro, levo and racemic isomer; the synthetic product is always optically inactive. The 1-terpineneol or 1-meththyl-4-isopropyl-3-cyclohexen-1-ol has been prepared by Wallach (Burdock, 1997).
Properties of Terpinen-4-ol
| Melting point: | 137-188 °C |
| Boiling point: | 88-90 °C |
| alpha | +25.2° |
| Density | 0.931 g/mL at 25 |
| refractive index | n |
| FEMA | 2248 | 4-CARVOMENTHENOL |
| Flash point: | 175 °F |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Liquid |
| pka | 14.94±0.40(Predicted) |
| color | Clear colorless to slightly yellow |
| Specific Gravity | 0.930.9265 (19℃) |
| Odor | at 100.00 %. pepper woody earth musty sweet |
| optical activity | [α]20/D 27°, neat |
| Water Solubility | Very slightly soluble |
| Merck | 3935 |
| JECFA Number | 439 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 562-74-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-(562-74-3) |
| EPA Substance Registry System | 4-Terpineol (562-74-3) |
Safety information for Terpinen-4-ol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Terpinen-4-ol
| InChIKey | WRYLYDPHFGVWKC-UHFFFAOYSA-N |
Terpinen-4-ol manufacturer
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![(2S)-2-[[[(1,1-Dimethylethyl)diphenylsilyl]oxy]methyl]-1,3-oxathiolan-5-ol 5-Acetate](https://img.chemicalbook.in/CAS/20180601/GIF/202532-88-5.gif)
You may like
-
 562-74-3 Terpinene-4-ol 98%View Details
562-74-3 Terpinene-4-ol 98%View Details
562-74-3 -
 Terpinene-4-ol 98%View Details
Terpinene-4-ol 98%View Details
562-74-3 -
 Terpinen-4-ol 98% (GC) CAS 562-74-3View Details
Terpinen-4-ol 98% (GC) CAS 562-74-3View Details
562-74-3 -
 Terpinen 4-ol CAS 562-74-3View Details
Terpinen 4-ol CAS 562-74-3View Details
562-74-3 -
 Terpene 4 Ol, 1 LView Details
Terpene 4 Ol, 1 LView Details
562-74-3 -
 Natural Aroma Terpinen-4-OL (99%) LiquidView Details
Natural Aroma Terpinen-4-OL (99%) LiquidView Details
562-74-3 -
 4-Carvomenthenol, Packaging Type: DrumView Details
4-Carvomenthenol, Packaging Type: DrumView Details
562-74-3 -
 TERPINEN-4-OLView Details
TERPINEN-4-OLView Details
562-74-3
