Terephthalic acid
Synonym(s):1,4-Benzenedicarboxylic acid, p-Phthalic acid;Benzene-1,4-dicarboxylic acid;Terephthalic acid
- CAS NO.:100-21-0
- Empirical Formula: C8H6O4
- Molecular Weight: 166.13
- MDL number: MFCD00002558
- EINECS: 202-830-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
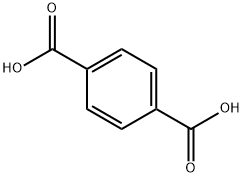
What is Terephthalic acid?
Description
Terephthalic acid (TPA), with the chemical formula $\text{C}_6\text{H}_4(\text{COOH})_2$, is a colorless organic solid. As a commodity chemical, its primary application is as a precursor in the synthesis of polyethylene terephthalate (PET), which is utilized in the production of textiles and plastic bottles. Annual production of TPA reaches several million tons. It is one of the three structural isomers of phthalic acid.
Chemical properties
Terephthalic acid is a white crystalline solid. Soluble in alkaline solution, slightly soluble in hot ethanol, insoluble in water, ether, glacial acetic acid and chloroform, consequently up until around 1970 most crude terephthalic acid was converted to the dimethyl ester for purification. It sublimates when heated.
History
Terephthalic acid gained prominence following the work of Winfield and Dickson in Britain around 1940. Prior research by Carothers and colleagues in the United States had established the synthesis of high molecular weight linear polyesters via the reaction of diacids and diols; however, their use of aliphatic diacids and diols resulted in polyesters unsuitable for fiber spinning. Winfield and Dickson subsequently discovered that symmetrical aromatic diacids yield materials that are high-melting, crystalline, and fiber-forming. Poly(ethylene terephthalate) (PET), derived from this discovery, has since become the most widely produced synthetic fiber by volume.
The Uses of Terephthalic acid
Terephthalic acid (TPA) is a benzenepolycarboxylic acid with potential anti-hemorrhagic properties. It is a high-tonnage chemical, widely used in the production of synthetic materials, notably polyester fibers (poly-(ethylene terephthalate)).
What are the applications of Application
Terephthalic acid is mainly used for the production of poly (ethylene terephthalate). Also production of plasticizer dioctyl phthalate (DOTP) and polyester plasticized agents. Terephthalic acid and polyhydric alcohols have a condensation reaction withd iethylene glycol, triethylene glycol, glycerol, propylene glycol, butylene glycol, etc. preparation of the polyester plasticizer.
Definition
ChEBI: Terephthalic acid is a benzenedicarboxylic acid carrying carboxy groups at positions 1 and 4. One of three possible isomers of benzenedicarboxylic acid, the others being phthalic and isophthalic acids. It is a conjugate acid of a terephthalate(1-).
Production Methods
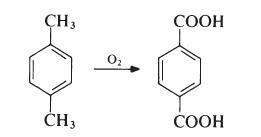
Terephthalic acid is produced by oxidation of p-xylene by oxygen in air:
This reaction proceeds through a p-toluic acid intermediate which is then oxidized to terephthalic acid. In p-toluic acid, deactivation of the methyl by the electron withdrawing carboxylic acid group makes the methyl one tenth as reactive as xylene itself, making the second oxidation significantly more difficult . The commercial process utilizes acetic acid as solvent and a catalyst composed of cobalt and manganese salts, with a bromide promoter.
What are the applications of Application
Virtually the entire world's supply of terephthalic acid and dimethyl terephthalate are consumed as precursors to polyethylene terephthalate (PET). World production in 1970 was around 1.75 million tones. By 2006, global purified terephthalic acid (PTA) demand had exceeded 30 million tonnes.
There is a smaller, but nevertheless significant, demand for terephthalic acid in the production of poly butylene terephthalate and several other engineering polymers.
Synthesis
Benzoic acid, phthalic acid and other benzene-carboxylic acids in the form of alkali-metal salts, comprise the chargestock. In a first step, the alkali-metal salts (usually potassium) are converted to terephthalates when heated to a temperature exceeding 350 °C (662 °F). The dried potassium salts (of benzoic acid or o- or isophthalic acid) are heated in anhydrous form to approximately 420 °C (788 °F) in an inert atmosphere (CO2) and in the presence of a catalyst (usually cadmium benzoate, phthalate, oxide, or carbonate). The corresponding zinc compounds also have been used as catalysts. In a following step, the reaction products are dissolved in H2O and the terephthalic acid precipitated out with dilute H2SO4. The yield of terephthalic acid ranges from 95 to 98%.
Preparation
The major commercial route to terephthalic acid which is suitable for the
direct preparation of poly(ethylene terephthalate) is from p-xylene:
p-Xylene is obtained largely from petroleum sources, being a product of the fractionation of reformed naphthas. The oxidation is carried out in the liquid phase. Typically, air is passed into a solution of p-xylene in acetic acid at about 200?? and 2 MPa (20 atmospheres) in the presence of a catalyst system containing cobalt and manganese salts and a source of bromide ions. The terephthalic acid produced contains only small amounts of impurities (mainly p-carboxybenzaldehyde), which are readily removed. The acid is dissolved in water at about 2500 e and 5 MPa (50 atmospheres) and treated with hydrogen (which converts the aldehyde to p-toluic acid). The solution is then cooled to 100?? and pure terephthalic acid crystallizes.
Synthesis Reference(s)
Chemistry Letters, 15, p. 299, 1986
Journal of the American Chemical Society, 82, p. 2876, 1960 DOI: 10.1021/ja01496a051
The Journal of Organic Chemistry, 44, p. 4727, 1979 DOI: 10.1021/jo00393a063
General Description
White powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Terephthalic acid (TPA) is a carboxylic acid. Its reaction with a base is an exothermic neutralization process, yielding water and a salt. Although TPA is largely insoluble in water, its ability to absorb atmospheric moisture and partially dissolve can induce corrosion in metallic containers, including those composed of iron, steel, and aluminum. TPA reacts with cyanide salts or solutions to release gaseous hydrogen cyanide. Exothermic reactions that generate flammable or toxic gases occur upon contact with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Similar heat-generating reactions occur with sulfites, nitrites, thiosulfates, and dithionites, also producing flammable or toxic gases. The reaction with carbonates and bicarbonates is exothermic, yielding carbon dioxide. TPA is reactive toward both strong oxidizing and reducing agents, with both processes generating heat. Furthermore, it may initiate polymerization reactions or function as a chemical catalyst.
Fire Hazard
Flash point data for Terephthalic acid are not available. Terephthalic acid is probably combustible.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by intravenous and intraperitoneal routes. Mildly toxic by ingestion. An eye irritant, Can explode during preparation. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
TPA is used primarily in the production of polyethylene terephthalate polymer for the fabrication of polyester fibers and films. A high-volume production chemical in the United States.
Purification Methods
Purify the acid via the sodium salt which, after crystallisation from water, is re-converted to the acid by acidification with mineral acid. Filter off the solid, wash it with H2O and dry it in a vacuum. The S-benzylisothiuronium salt has m 204o (from aqueous EtOH). [Beilstein 9 IV 3301.]
Incompatibilities
Combustible; dust may form an explosive mixture with air. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Terephthalic acid
| Melting point: | >300 °C (lit.) |
| Boiling point: | 214.32°C (rough estimate) |
| Density | 1.58 g/cm3 at 25 °C |
| vapor pressure | <0.01 mm Hg ( 20 °C) |
| refractive index | 1.5100 (estimate) |
| Flash point: | 260°C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | 15mg/l (experimental) |
| form | Crystalline Powder |
| pka | 3.51(at 25℃) |
| color | White |
| PH | 3.36(1 mM solution);2.79(10 mM solution);2.26(100 mM solution) |
| Water Solubility | slightly soluble in water (0,017 g/L at 25°C) |
| Merck | 14,9162 |
| BRN | 1909333 |
| Exposure limits | ACGIH: TWA 10 mg/m3 |
| Dielectric constant | 1.5(Ambient) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 100-21-0(CAS DataBase Reference) |
| EPA Substance Registry System | Terephthalic acid (100-21-0) |
Safety information for Terephthalic acid
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P321:Specific treatment (see … on this label). P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Terephthalic acid
Terephthalic acid manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Purified Terephthalic Acid (PTA) 98%View Details
Purified Terephthalic Acid (PTA) 98%View Details -
 Terephthalic Acid CAS 100-21-0View Details
Terephthalic Acid CAS 100-21-0View Details
100-21-0 -
 Terephthalic acid 95% CAS 100-21-0View Details
Terephthalic acid 95% CAS 100-21-0View Details
100-21-0 -
 Terephthalic acid, 99%+ CAS 100-21-0View Details
Terephthalic acid, 99%+ CAS 100-21-0View Details
100-21-0 -
 Terephthalic acid CAS 100-21-0View Details
Terephthalic acid CAS 100-21-0View Details
100-21-0 -
 Terephthalic acid CAS 100-21-0View Details
Terephthalic acid CAS 100-21-0View Details
100-21-0 -
 TEREPHTHALIC ACID Pure CAS 100-21-0View Details
TEREPHTHALIC ACID Pure CAS 100-21-0View Details
100-21-0 -
 Terephthalic acid CAS 100-21-0View Details
Terephthalic acid CAS 100-21-0View Details
100-21-0
