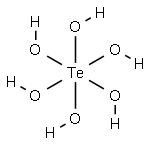
Tellurous acid
- Empirical Formula: H2O3Te
- Molecular Weight: 177.61408
- Update Date: 2024-07-11 14:13:35

What is Tellurous acid?
Description
H2TeO3 has been synthesized as a solid but it is not
well characterized. If tellurium dioxide, TeO2, is added
to water, tellurous acid does not easily form, but it can
be made other ways. Its CAS number is 10049-23-7.
Tellurous acid can be formed from the chlorides. Two
chlorides are known, the dichloride, TeCl2, and the tetrachloride,
TeCl4. They are both obtained by passing chlorine
over tellurium, the product being separated by
distillation (the tetrachloride is the less volatile). The
dichloride is an amorphous, readily fusible, almost black
solid. It is decomposed by water with formation of tellurium
and tellurous acid:
2TeCl2+ 3H2O ? Te+H2TeO3+ 4HCl
However, pure tellurous acid, H2TeO3, can be
obtained when the tetrachloride is decomposed by
water, or on dissolving tellurium in nitric acid and pouring
the solution into water. It is a colorless solid and
behaves as a dibasic acid. The alkali tellurites are soluble
in water. They also gives rise to super acid salts, such as
KHTeO3·H2TeO3.
Chemical properties
White, crystalline powder. Soluble in dilute acids, alkalies; slightly soluble in water, alcohol.
Hazard
As for tellurium.
Properties of Tellurous acid
| pka | 2.5(at 25℃) |
Safety information for Tellurous acid
Computed Descriptors for Tellurous acid
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

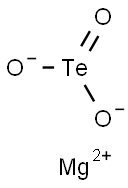
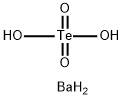
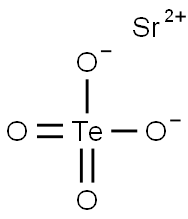
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1