Teicoplanin
Synonym(s):Targocid;Teichomycin;Teicoplanin
- CAS NO.:61036-62-2
- Empirical Formula: C78H77Cl2N8O32R
- Molecular Weight: 1709.39
- MDL number: MFCD34185384
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 21:30:20
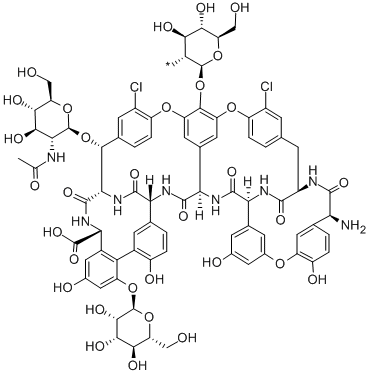
What is Teicoplanin?
Absorption
Teicoplanin is poorly absorbed after oral administration but is 90% bioavailable when administered intramuscularly.
Description
Teicoplanin is a new antibiotic, the second glycopeptide to be developed in over 30 years. Compared with vancomycin, the only such agent currently available, teicoplanin is equiefficacious, and has milder side-effects and a longer half-life, allowing once-daily dosing and bolus injection. Teicoplanin is claimed to have an overall cure rate of 92% in infections involving skin, joint and bone, endocaditis and septicemia.
Chemical properties
Yellowish, amorphous powder.
Originator
Merrell Dow (Italy)
The Uses of Teicoplanin
Teicoplanin complex is family of closely related metabolites produced by Actinoplanes teichomyceticus. Teicoplanin complex possesses potent broad spectrum antibiotic activity against Gram positive bacteria, including MRSA and E. faecalis. The metabolites share a common glycopeptide core (teicoplanin A-3) on which a family of fatty acids varying in length, degree of saturation and branching are linked as amides through one of the aminoglycoside moieties. The major component of the complex is teicoplanin A2 which itself has five major components (teicoplanin A2-1 to A2-5) and four minor components (teicoplanin RS-1 to RS-4).
The Uses of Teicoplanin
anticonvulsant, antipsychotic
The Uses of Teicoplanin
antidepressant
Background
Teicoplanin is a glycopeptide antibiotic consisting of a mixture of several compounds, five major (named teicoplanin A2-1 through A2-5) and four minor (named teicoplanin RS-1 through RS-4). All teicoplanins share a same glycopeptide core, teicoplanin A3-1, but differ in the length and conformation of side chains attached to their β-D-glucosamine moiety.
Indications
For the treatment of bacterial infections caused by susceptible microorganisms.
What are the applications of Application
Teicoplanin Complex is a complex of broad-spectrum antibiotics
brand name
Targocid
General Description
Teicoplanin (Teichomycin A2, Targocid) is a mixture offive closely related glycopeptide antibiotics produced bythe actinomycete Actinoplanes teichomyceticus. The teicoplanin factors differ only in the acyl group inthe northernmost of two glucosamines glycosidicallylinked to the cyclic peptide aglycone. Another sugar, Dmannose,is common to all of the teicoplanins. Thestructures of the teicoplanin factors were determined independentlyby a combination of chemical degradationand spectroscopic methods in three different groupsin 1984.
The teicoplanin complex is similar to vancomycin structurallyand microbiologically but has unique physical propertiesthat contribute some potentially useful advantages.While retaining excellent water solubility, teicoplanin has significantlygreater lipid solubility than vancomycin. Thus, teicoplaninis distributed rapidly into tissues and penetratesphagocytes well. The complex has a long elimination halflife,ranging from 40 to 70 hours, resulting from a combinationof slow tissue release and a high fraction of protein bindingin the plasma (~90%). Unlike vancomycin, teicoplanin isnot irritating to tissues and may be administered by intramuscularor intravenous injection. Because of its long half-life, teicoplaninmay be administered on a once-a-day dosing schedule.Orally administered teicoplanin is not absorbedsignificantly and is recovered 40% unchanged in the feces.
Teicoplanin exhibits excellent antibacterial activity againstGram-positive organisms, including staphylococci, streptococci,enterococci, Clostridium and Corynebacterium spp.,Propionibacterium acnes, and L. monocytogenes. It is not activeagainst Gram-negative organisms, including Neisseriaand Mycobacterium spp. Teicoplanin impairs bacterial cellwall synthesis by complexing with the terminal D-alanine-Dalaninedipeptide of the peptidoglycan, thus preventing crosslinkingin a manner entirely analogous to the action ofvancomycin.
Pharmacokinetics
It is a glycopeptide antiobiotic extracted from Actinoplanes teichomyceticus, with a similar spectrum of activity to vancomycin. Its mechanism of action is to inhibit bacterial cell wall synthesis. Oral teicoplanin has been demonstrated to be effective in the treatment of pseudomembranous colitis and Clostridium difficile-associated diarrhoea, with comparable efficacy to vancomycin.
Clinical Use
Antibacterial agent.
Metabolism
Two metabolites (metabolites 1 and 2; 2 to 3% of total teicoplanin) have been isolated after intravenous administration of radiolabeled teicoplanin. After purification, their structures were found to be new teicoplanin-like molecules, bearing 8-hydroxydecanoic and 9-hydroxydecanoic acyl moieties. This metabolic transformation is likely due to hydroxylation in the omega-2 and omega-1 positions for metabolites 1 and 2, respectively, of the C-10 linear side chain of component A2-3. This might explain the low extent of metabolism of teicoplanin if we consider that only component A2-3 has a linear chain that is susceptible to such oxidation.
Metabolism
Teicoplanin is excreted almost entirely by glomerular filtration in the urine, as unchanged drug. Two metabolites are formed probably by hydroxylation and represents 2-3% of the administered dose. Unchanged teicoplanin is mainly excreted by the urinary route while 2.7% of the administered dose is recovered in feces (via bile excretion) within 8 days following administration.
Properties of Teicoplanin
| Melting point: | 310 °C (decomp) |
| RTECS | WY1559000 |
| storage temp. | 2-8°C |
| solubility | H2O: soluble10mg/mL |
| form | powder |
| color | white to faint yellow |
| Water Solubility | Sparingly soluble in water |
| CAS DataBase Reference | 61036-62-2 |
Safety information for Teicoplanin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Teicoplanin
Teicoplanin manufacturer
Sumar biotech LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 61036-62-2 Teicoplanin 98%View Details
61036-62-2 Teicoplanin 98%View Details
61036-62-2 -
 61036-62-2 98%View Details
61036-62-2 98%View Details
61036-62-2 -
 Teicoplanin 61036-62-2 98%View Details
Teicoplanin 61036-62-2 98%View Details
61036-62-2 -
 61036-62-2 Teicoplanin 98%View Details
61036-62-2 Teicoplanin 98%View Details
61036-62-2 -
 Teicoplanin CAS 61036-62-2View Details
Teicoplanin CAS 61036-62-2View Details
61036-62-2 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
