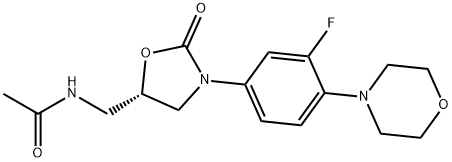
Linezolid
Synonym(s):Linezolid;N-[[(5S)-3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide;PNU-100766;U-100766
- CAS NO.:165800-03-3
- Empirical Formula: C16H20FN3O4
- Molecular Weight: 337.35
- MDL number: MFCD00937825
- EINECS: 605-416-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
What is Linezolid?
Absorption
Linezolid is extensively absorbed following oral administration and has an absolute bioavailability of approximately 100%. Maximum plasma concentrations are reached within approximately 1 to 2 hours after dosing (Tmax) and range from 8.1-12.9 mcg/mL after single doses and 11.0-21.2 mcg/mL after multiple dosing.
The absorption of orally administered linezolid is not significantly affected by co-administration with food and it may therefore be given without regard to the timing of meals.
Toxicity
Clinical signs of overdosage observed in rats were decreased activity and ataxia (2000 mg/kg/day) and in dogs were vomiting and tremors (3000 mg/kg/day). Treatment of overdose should involve symptomatic and supportive measures and may include hemodialysis if clinically necessary.
Description
Linezolid reached the US market for the treatment of patients with infections caused by serious Gram-positive pathogens, particularly skin and soft tissue infections, communityacquired pneumonia and vancomycin-resistant enterococcal infections. Linezolid is the (S)-enantiomer of an oxazolidin-2-one synthesized in a multistep process from 3,4- difluoronitrobenzene, the key step being the cyclization of a carbamate, using a chiral epoxyester, into an enantiomerically pure oxazolidin-2-one. Linezolid can be considered as the first of a new class of antibacterial agents known as oxazolidinones, its mechanism of action being related to the inhibition of early ribosomal protein synthesis without directly inhibiting DNA or RNA synthesis. In vitro studies demonstrated that linezolid was effective, at potency levels similar to vancomycin, against staphylococcal, streptococcal and pneumococcal infections (MIC values in the range of 0.5 to 2 μg/ml), enterococcal species including VRE and VSE (MIC values about 4 μg/ml), but also other vancomycin-resistant bacteria. Linezolid is rapidly absorbed orally, its bioavailability is nearly complete at 250 mg dose giving a Cmax to MIC ratio sufficient to have pathogenic strain eradication in the clinical setting. It is considered that this new promising agent may offer new options for therapy of multi-drug infections.
Description
Linezolid (trade name Zyvox) is a synthetic antibiotic that was developed by the Upjohn Co. in the late 1990s. It was approved by the US Food and Drug Administration in 2000 and is now one of the top-selling antibiotics in the world. Linezolid is used to treat methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and other Gram-positive pathogenic bacteria.
Linezolid is relatively safe, but it can cause diarrhea, headache, nausea, and vomiting. Like other antibiotics, its ability to suppress a wide range of bacteria may lead to fungal infections.
Chemical properties
White Solid
Originator
Pharmacia Corp. (US)
The Uses of Linezolid
Linezolid has been used in checkerboard-type drug combination MIC (minimum inhibitory concentrations) assay to determine whether ivacaftor shows positive interaction with linezolid. It has been used for the determination of minimum biofilm inhibitory concentration and for the treatment of Mycobacterium abscessus-infected Drosophila melanogaster w1118 flies.
The Uses of Linezolid
Prototype of the oxazolidinone antimicrobials; inhibits bacterial mRNA translation.
Indications
Linezolid is indicated in adults and children for the treatment of infections caused by susceptible Gram-positive bacteria, including nosocomial pneumonia, community-acquired pneumonia, skin and skin structure infections, and vancomycin-resistant Enterococcus faecium infections. Examples of susceptible bacteria include Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, and Streptococcus agalactiae.
Linezolid is not indicated for the treatment of Gram-negative infections, nor has it been evaluated for use longer than 28 days.
Background
Linezolid is a synthetic antibiotic that is used for the treatment of infections caused by aerobic Gram-positive bacteria. Its effects are bacteriostatic against both enterococci and staphylococci and bactericidal against most isolates of streptococci. Linezolid exerts its antibacterial activity by inhibiting the initiation of bacterial protein synthesis - more specifically, it binds to the 23S ribosomal RNA of the 50S subunit and, in doing so, prevents the formation of the 70S initiation complex, which is essential for bacterial reproduction. Linezolid was approved by the US Food and Drug Administration (FDA) in April 2000, after which it was approved in Canada and South America. In Europe, linezolid was approved in the UK in February 2001, Switzerland, and several other non-Union European countries.
Definition
ChEBI: An organofluorine compound that consists of 1,3-oxazolidin-2-one bearing an N-3-fluoro-4-(morpholin-4-yl)phenyl group as well as an acetamidomethyl group at position 5. A synthetic antibacterial agent that inhibits bacterial protein synt esis by binding to a site on 23S ribosomal RNA of the 50S subunit and prevents further formation of a functional 70S initiation complex.
Manufacturing Process
N-Carbobenzoxy-3-fluoro-4-morpholinylaniline (3.00 mmol, 1.000 eq) and
tetrahydrofuran (3.5 ml) were agitated and cooled. The lithium t-amylate
mixture [prepared in THF at 25°C from t-amyl alcohol (0.66 ml, 6.03 mmol,
2.00 eq) and butyl lithium (1.8 ml, 2.5 M in hexanes, 4.55 mmol, 1.5 eq)] is
then added to the carbamate mixture at less than 8°C and rinsed in with THF
(1 ml).
Tetrahydrofuran (3.2 ml) and S-(+)-3-chloro-1,2-propanediol (0.299 ml, 3.58
mmol, 1.19 eq) are mixed. The mixture of THF (3.2 ml) and S-(+)-3-chloro-
1,2-propanediol (0.299 ml, 3.58 mmol, 1.19 eq) is cooled to -16°C and
potassium t-butoxide (3.2 ml, 1.0 M) in THF (3.2 mmol, 1.07 eq) is added at
less than -10°C. The resulting slurry is stirred at -14-0°C for 1 hour. Then
added to the lithium anion mixture while maintaining both mixtures at 0°C,
then rinsed in with THF (2 ml). The resultant slurry is stirred at 20-23°C for 2
hour and then cooled to 6°C and a mixture of citric acid monohydrate (0.4459
g, 2.122 mmol, 0.705 eq) in water (10 ml) is added. The resultant liquid
phases are separated and the lower aqueous phase is washed with ethyl
acetate (12 ml). The organic layers are combined and solvent is removed
under reduced pressure until a net weight of 9.73 g remains. Heptane (10 ml)
and water (5 ml) are added and solvent is removed 4-nitrobenzenesulfonyl
chloride y reduced pressure until a total volume of 5 ml remains. The
precipitated product is collected by vacuum filtration and washed with water
(7 ml). The solids are dried in a stream of nitrogen to give (R)-[N-3-(3-fluoro-
4-(4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methanol.
To a slurry of (R)-[N-3-(3-fluoro-4-(4-morpholinylphenyl)-2-oxo-5-
oxazolidinyl]methanol (43.0 g, 145 mmol) and triethylamine (36 g, 355
mmol) in methylene chloride (450 ml) at 0°C is added a mixture of 4-
nitrobenzenesulfonyl chloride (32 g, 145 mmol) in methylene chloride (55 ml).
The mixture is stirred in a 0°C bath for 30 min and then quenched with
hydrochloric extracted again with methylene chloride (200 ml). The combined
organic extracts are then concentrated column chromatographed (silica gel,
methanol/methylene chloride 1-2/98-99, about 8 L). The appropriate fractions
are combined and concentrated to give the (R)-[N-3-(3-fluoro-4-(4-
morpholinylphenyl)-2-oxo-5-oxazolidinyl]methanol 4-nitrobenzenesulfonate
ester.
A mixture of (R)-[N-3-(3-fluoro-4-(4-morpholinylphenyl)-2-oxo-5-
oxazolidinyl]methanol 4-nitrobenzenesulfonate ester, isopropanol (149 ml),
acetonitrile (245 ml), salicylaldehyde (13.7 ml, 129 mmol) and aqueous
ammonia (30%, 257 ml, 4.02 mol), is heated to 40°C and stirred at 39-42°C
for 24 hours. The mixture is then cooled to -22°C and the precipitate collected
by vacuum filtration, washed with water (10 ml) and dried to give the (S)-[N-
3-(3-fluoro-4-(4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methylamine
salicylaldehyde imine.
(S)-[N-3-(3-Fluoro-4-(4-morpholinylphenyl)-2-oxo-5-oxazolidinyl] methylaminesalicylaldehyde imine (1.0068 g, 2.521 mmol) is slurried in water
(10 ml) and 37% aqueous hydrochloric acid (0.417 ml, 5.04 mmol) and
stirred at 20-25°C for 15 hours. Toluene (10 ml) is added and the phases
separated; then, the organic phase is washed with hydrochloric acid (1 M, 5
ml) and the combined aqueous phases are washed with toluene (10 ml). The
toluene wash is back-extracted with hydrochloric acid (1 M, 5 ml). The
combined aqueous phases are then adjusted to pH 13.0 with aqueous sodium
hydroxide (50%, 1.83 g, 22.9 mmol). To the resultant slurry is then added
methylene chloride (10 ml) and sodium chloride (1 g) and the phases
separated. The aqueous phase is then washed with methylene chloride (10
ml). To the combined organic phases is then added acetic anhydride (0.472
ml, 5.00 mmol) while maintaining 24-27°C. The mixture is stirred 40 min,
then water is added (5 ml). The phases are separated and the aqueous phase
is washed with methylene chloride (5 ml). The combined organic phases are
concentrated and ethyl acetate (25 ml) is added. The mixture is warmed to
70°C and then the resultant mixture is slowly cooled to -25°C. The precipitate
is collected by vacuum filtration, washed with ethyl acetate (5 ml) and dried
to give the (S)-[[N-3-(3-fluoro-4-(4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]
methyl]acetamide, HPLC major component (99.93 area % at 254 nm
detection) retention time = 0.97 min, column Zorbax RX-C8, mobile phase
650 ml acetonitrile, 1.85 ml triethylamine, 1.30 ml acetic acid and sufficient
water to make 1000 ml; flow rate = 3 ml/min.
brand name
Zyvox (Pharmacia & Upjohn).
Therapeutic Function
Antibacterial
Antimicrobial activity
It exhibits potent activity against a wide range of Gram-positive organisms, including those that are resistant to other antimicrobial agents. Methicillinresistant Staph. aureus and coagulase-negative staphylococci are susceptible, as are enterococci, including vancomycin-resistant Enterococcus faecalis and Ent. faecium. Penicillin-sensitive and resistant isolates of Streptococcus pneumoniae are equally susceptible. Less common Gram-positive pathogens are also susceptible; the minimum inhibitory concentrations (MICs) for Bacillus spp., Corynebacterium spp., Listeria monocytogenes, Aerococcus spp., Micrococcus spp. and Rhodococcus equi are all ≤2 mg/L. M. tuberculosis is susceptible, with typical MICs ≤1 mg/L for sensitive and multidrug- resistant strains.
All enterobacteria, Pseudomonas spp. and other non-fermentative aerobic Gram-negative bacilli, including Acinetobacter spp., are resistant. Moraxella catarrhalis, Legionella spp.,Mycoplasma spp. and Chlamydia spp. are inhibited by 4–8 mg/L. Activity against Haemophilus influenzae is modest.
Among anaerobes, Clostridium perfringens and Peptostreptococcus spp. are inhibited by <2 mg/L. Typical MICs (mg/L) for Gram-negative anaerobes include: Bacteroides spp., 4–8; Prevotella spp., 1–4; Fusobacterium spp., 0.125–1.
Activity is bacteristatic against most susceptible species, but modest bactericidal activity has been demonstrated against some strains of Str. pneumoniae, C. perfringens and Bacteroides fragilis. Inhibition of toxin production by staphylococci and streptococci in the presence of sub-MIC concentrations has been described.
Linezolid may antagonize the bactericidal action of some antibiotics (e.g. gentamicin). No evidence of synergy has been found in various experimental systems with gentamicin against vancomycin-resistant Enterococcus spp. or with vancomycin, gentamicin, ciprofloxacin, fusidic acid or rifampicin (rifampin) against methicillin-resistant Staph. aureus.
Acquired resistance
Isolates of Staph. aureus and E. faecalis for which the MIC of linezolid is raised have been obtained following serial exposure to gradients of the drug. However, induction of resistance requires many passages over several weeks. Resistance in these laboratory mutants is associated with modifications of the 23S rRNA gene.
Overall, resistance rates in clinical isolates are very low at <0.5%. Resistance is reported primarily in coagulase-negative staphylococci (1.77%) and enterococci (1.13%; mostly E. faecium), with exceptionally low resistance rates in Staph. aureus (0.06%). Risk factors for emergence of resistance include prolonged use of the drug, the presence of irremovable indwelling devices, sequestered sites of infection and low-dose therapy for infections caused by vancomycin-resistant enterococci or methicillin-resistant Staph. aureus. Resistance in clinical isolates is most often associated with gene mutations in which guanosine is replaced by uracil in the 23S rRNA. Nosocomial clonal spread of such mutants has been described in coagulase-negative staphylococci and enterococci. Resistance conferred by a novel mobile element, cfr, has been described in two isolates of staphylococci.
General Description
Linezolid (Zyvox) is an oxazolidinedione-type antibacterialagent that inhibits bacterial protein synthesis. It acts in theearly translation stage, preventing the formation of a functionalinitiation complex. Linezolid binds to the 30S and 70Sribosomal subunits and prevents initiation complexes involvingthese subunits. Collective data suggest that the oxazolidindionespartition their ribosomal interaction between thetwo subunits. Formation of the early tRNAfMet-mRNA-70Sor 30S is prevented. Linezolid is a newer synthetic agent, andhence, cross-resistance between the antibacterial agent andother inhibitors of bacterial protein synthesis has notbeen seen.
Linezolid possesses a wide spectrum of activity againstGram-positive organisms, including MRSA, penicillin-resistantpneumococci, and vancomycin-resistant Enterococcusfaecalis and E. faecium. Anaerobes such as Clostridium,Peptostreptococcus, and Prevotella spp. are sensitive tolinezolid.Linezolid is a bacteriostatic agent against most susceptibleorganisms but displays bactericidal activity against somestrains of pneumococci, B. fragilis, and Clostridiumperfringens.The indications for linezolid are for complicated anduncomplicated skin and soft-tissue infections, communityandhospital-acquired pneumonia, and drug-resistant Grampositiveinfections.
Pharmaceutical Applications
A synthetic oxazolidinone available for oral or intravenous administration. Soluble in water at a pH range of 5–9. Aqueous solutions (2 g/L) are stable at 25°C, 4°C and ?20°C for at least 3 months.
Biological Activity
Oxazolidinone antibiotic. Inhibits bacterial protein synthesis prior to chain initiation. Displays potent antibacterial activity against a variety of multidrug-resistant gram-positive microbes in vitro and in vivo .
Biochem/physiol Actions
Linezolid is an oxazolidinone antimicrobial. It binds to a site on the bacterial 23S ribosomal RNA of the 50S subunit and prevents the formation of a functional 70S initiation complex, thus inhibiting bacterial mRNA translation. Linezolid is also a weak, reversible, nonselective inhibitor of monoamine oxidase.
Mechanism of action
The mechanism of action is inhibition of protein synthesis, but at a stage different from that of other protein synthesis inhibitors. The oxazolidinones bind to the 23S rRNA of the 50S subparticle to prevent formation of a functional 70S initiation complex. It is considered to be bacteriostatic against enterococci and staphylococci but bacteriocidal against streptococci. Resistance to oxazolidinones is encountered in the clinic because of a mutation in the 23S rRNA. This is believed to distort the linezolid binding site. Gram-negative microorganisms are intrinsically resistant to linezolid because of the presence of endogenous efflux pumps that keep it from accumulating in the cells.
Pharmacokinetics
Oral absorption: >95%
Cmax 400 mg oral: 11–12 mg/L after 1–2 h
600 mg oral :18–21 mg/L after 1–2 h
600 mg intravenous: >15 mg/L after 1 h
Plasma half-life: c. 5.5 h
Volume of distribution :45–50 L
Plasma protein binding: 31%
absorption
Bioavailability after oral administration is almost complete. Plasma trough concentrations following oral doses of 400 mg and 600 mg every 12 h are >3.0 and >4.0 mg/L, respectively. With the higher dose, administered orally or intravenously, plasma concentrations remain above the MIC for most susceptible species throughout a 12 h dosage interval. After administration with high fat content food the maximum serum concentration achieved is lower and the peak delayed, but the area under the concentration–time curve (AUC) is unaltered.
Distribution
Linezolid is distributed widely in tissues and fluids. In human volunteers, maximum concentrations in inflammatory blister fluid averaged over 16 mg/L, with a mean penetrance of 104%. In patients undergoing hip arthroplasty, linezolid rapidly penetrates into bone, fat and muscle, achieving levels in excess of the MICs for susceptible organisms, with therapeutic concentrations maintained in the perioperative site hematoma fluid for more than 16 h. Mean penetration of linezolid into inflamed diabetic foot infection tissue is 101%, producing a concentration of 9.6 μg/g. Studies with human volunteers have also indicated good concentrations in pulmonary alveolar fluid with a mean fluid to plasma ratio of 3.2:1. When the meninges are not inflamed, the concentration in cerebrospinal fluid (CSF) is lower than that of plasma, with a CSF:plasma ratio of approximately 0.7:1. The concentration in sweat is about half that of plasma.
Other sites at which local concentrations exceed corresponding plasma concentrations, based on animal studies, include kidney, adrenal, liver and gastrointestinal tract. In a rat model of endocarditis, heart valve tissue and plasma concentrations were approximately equivalent.
Pharmacokinetic properties are unaltered in elderly patients and dose adjustment is unnecessary. Single-dose pharmacokinetic studies indicate that plasma clearance and volume of distribution are greater in children than in adults, while peak and trough serum concentrations are lower. Shorter dosing intervals (every 8 h) are therefore recommended for most therapeutic indications in children.
Metabolism
Linezolid undergoes non-renal as well as renal metabolism. Non-renal metabolism is by slow chemical oxidation in a process that does not discernibly interact with the hepatic cytochrome P450 system. The oxidants contributing to metabolism of the drug have not yet been fully elucidated, but in-vivo studies suggest the process is mediated by reactive oxygen species produced throughout the body. The metabolites produced following non-renal metabolism are an aminoethoxyacetic acid and a hydroxyethylglycine metabolite, neither of which has any significant antimicrobial activity. Non-renal clearance rates are 120 mL/min and account for almost 65% of total body clearance. Since it does not appear to act as an inducer or inhibitor of cytochrome P450 enzymes, interactions with drugs metabolized by these enzymes are unlikely to occur.
excretion
Renal clearance accounts for approximately 50 mL/min of the total body clearance of 170 mL/min. Under steady-state conditions, approximately 30% of the dose is excreted unchanged in the urine.
In populations with varying degrees of renal function (creatinine clearance range of 10–>80 mL/min) there is no evidence of alteration in total body clearance, and adjustment of dose in patients with renal insufficiency is not recommended. However, accumulation of metabolites, up to 10-fold, occurs in patients with severe renal impairment (creatinine clearance <30 mL/min). The clinical significance of this is unknown, but linezolid should be used with caution in patients with severe renal impairment. Approximately one-third of the dose is removed by hemodialysis and since total apparent clearance is increased during dialysis, one of the 12-hourly doses should be administered after the procedure. Accumulation of metabolites also occurs in patients on dialysis, with unknown clinical significance, and caution in use in hemodialysis is advised. There are no available data on pharmacokinetics in patients undergoing peritoneal dialysis or hemofiltration.
In patients with mild to moderate hepatic impairment there is no significant change to the pharmacokinetic profile. Accordingly, dosage adjustment is not recommended in patients with mild to moderate liver disease. The pharmacokinetics of linezolid in severe hepatic failure have not been studied, but as its metabolism is predominantly non-enzymatic, the pharmacokinetics would not be expected to alter significantly.
Clinical Use
Linezolid is primarily used for the treatment of infections caused, or likely to be caused, by methicillin-resistant Staph. aureus, vancomycin-resistant enterococci and penicillin resistant Str. pneumoniae. Combination therapy with an antimicrobial active against Gram-negative bacteria is indicated if concomitant infection with a Gram-negative pathogen is suspected or confirmed.
Outside of licensed indications, it has been used in the treatment of bone and joint infections, endocarditis, central nervous system infections, infections in neutropenic patients and drug-resistant tuberculosis.
Side Effects
Most reported adverse events are mild or moderate, with reactions severe enough to lead to withdrawal of therapy occurring in less than 3% of patients.The most frequent side effects are gastrointestinal disturbances (diarrhea, nausea, vomiting and taste alteration) and headache. The reported incidence of Clostridium difficile complications is 0.2%.
Mild and transient abnormalities of liver function tests
(elevation of transaminases and/or alkaline phosphatase) occur in more than 1% of patients. Skin reactions, including rashes, dermatitis, pruritus and diaphoresis, are uncommon.
Serious but infrequent adverse drug effects include myelosuppression, peripheral neuropathy, optic neuropathy and lactic acidosis. These adverse events, which probably result from inhibition of mitochondrial protein synthesis, occur primarily in patients treated for >28 days. Myelosuppression
generally occurs only after more than 2 weeks of treatment and increases with longer durations. It occurs more frequently in patients with severe renal insufficiency and is reversible on discontinuation of therapy.
Reversal of cytopenias by concomitant administration of vitamin B6 has been described. Weekly monitoring of full blood count is recommended for all patients, with more frequent monitoring of those in the following categories: pre-existing anemia or thrombocytopenia; receiving concomitant drugs that may cause anemia or thrombocytopenia; severe renal insufficiency; treatment for more than 10–14 days.
Peripheral and optic neuropathy are serious but infrequent. Most cases are associated with treatment for more than 28 days (median 5 months), but neuropathies have occurred with shorter courses. In most cases optic neuropathy improved or resolved on cessation of therapy but peripheral neuropathy did not.
Lactic acidosis can occur within a week of commencing therapy but is most often seen in patients receiving prolonged treatment (median 6 weeks).
Linezolid is a weak, reversible monoamine oxidase inhibitor (MAOI) with potential interaction with adrenergic and serotonergic drugs. Co-administration of sympathomimetics, vasopressors or dopaminergic agents may lead to an enhanced pressor response. It should be co-administered with these drugs only under conditions where close observation and monitoring of blood pressure is available, and their initial doses should be reduced and then titrated to achieve the desired pressor effect. Similarly, concomitant administration of linezolid with agents that increase central nervous system serotonin concentrations can lead to serotonin toxicity (serotonin syndrome). This most commonly follows concurrent administration of selective serotonin receptor inhibitors, but can occur with tricyclic antidepressants or any MAOI. Since MAOIs and their active metabolites have long elimination half-lives, linezolid is contraindicated in patients who are taking these drugs or have taken them in the previous 2 weeks.
in vitro
linezolid was a potent inhibitor of cell-free transcription-translation in e. coli. ic50 was 1.8 mm[3]. linezolid mics vary slightly owing to the different test method and laboratory. the mic values were between 0.5 and 4 mg/l for streptococci, enterococci and staphylococci [4].
Cautions
Linezolid is an oxazolidinone antibacterial agent effective against most strains of aerobic Gram-positive bacteria and mycobacteria. It appears to be bacteriostatic against both staphylococci and enterococci and bactericidal against most isolates of streptococci. Linezolid has shown some in vitro activity against Gram-negative and anaerobic bacteria but is not considered efficacious against these organisms. Linezolid is a reversible and non-selective inhibitor of monoamine oxidase (MAO) enzymes. It can, therefore, contribute to the development of serotonin syndrome when administered alongside serotonergic agents such as selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants (TCAs). Linezolid should not be used to treat catheter-related bloodstream infections or catheter-site infections, as the risk of therapy appears to outweigh its benefits under these circumstances.
Metabolism
Linezolid is primarily metabolized to two inactive metabolites: an aminoethoxyacetic acid metabolite (PNU-142300) and a hydroxyethyl glycine metabolite (PNU-142586), both of which are the result of morpholine ring oxidation. The hydroxyethyl glycine metabolite - the most abundant of the two metabolites - is likely generated via non-enzymatic processes, though further detail has not been elucidated.
While the specific enzymes responsible for the biotransformation of linezolid are unclear, it does not appear to be subject to metabolism via the CYP450 enzyme system, nor does it meaningfully inhibit or induce these enzymes. Linezolid is, however, a reversible and non-selective inhibitor of monoamine oxidase enzymes.
Metabolism
Linezolid is well absorbed orally and is generally well tolerated; however, some severe cases of reversible blood dyscrasias have been noted, resulting in a package insert warning that complete blood counts should be monitored weekly, especially in patients with poorly draining infections and who are receiving prolonged therapy with the drug. Some interference with monoamine oxidase action has been seen, so patients should be cautious about ingesting tyramine-rich foods. Coadministration with adrenergic and serotonergic agents also is unadvisable. Additionally, lactic acidosis has been reported in patients receiving linezolid. Significant oxidative metabolism of the morpholine ring occurs but is not caused by cytochrome P450, so it does not interfere with other drugs metabolized by this system.
Storage
Room temperature
References
[1] tsiodras s, gold h s, sakoulas g, et al. linezolid resistance in a clinical isolate of staphylococcus aureus[j]. the lancet, 2001, 358(9277): 207-208.
[2] swaney s m, aoki h, ganoza m c, et al. the oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria[j]. antimicrobial agents and chemotherapy, 1998, 42(12): 3251-3255.
[3] shinabarger d l, marotti k r, murray r w, et al. mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions[j]. antimicrobial agents and chemotherapy, 1997, 41(10): 2132-2136.
[4] livermore d m. linezolid in vitro: mechanism and antibacterial spectrum[j]. journal of antimicrobial chemotherapy, 2003, 51(suppl 2): ii9-ii16.
[5] stalker d j, jungbluth g l. clinical pharmacokinetics of linezolid, a novel oxazolidinoneantibacterial[j]. clinical pharmacokinetics, 2003, 42(13): 1129-1140.
[6] clemett d, markham a. linezolid[j]. drugs, 2000, 59(4): 815-827.
[7] wunderink r g, cammarata s k, oliphant t h, et al. continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia[j]. clinical therapeutics, 2003, 25(3): 980-992.
Properties of Linezolid
| Melting point: | 176-1780C |
| Boiling point: | 585.5±50.0 °C(Predicted) |
| alpha | D20 -9° (c = 0.919 in chloroform) |
| Density | 1.302±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: >20mg/mL |
| pka | 15.53±0.46(Predicted) |
| form | powder |
| color | white to off-white |
| CAS DataBase Reference | 165800-03-3(CAS DataBase Reference) |
Safety information for Linezolid
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. |
Computed Descriptors for Linezolid
| InChIKey | TYZROVQLWOKYKF-ZDUSSCGKSA-N |
Linezolid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Linezolid 98%View Details
Linezolid 98%View Details -
 Linezolid Form II 99%View Details
Linezolid Form II 99%View Details -
 Linezolid 98%View Details
Linezolid 98%View Details -
 Linezolid 95% CAS 165800-03-3View Details
Linezolid 95% CAS 165800-03-3View Details
165800-03-3 -
 Linezolid 99% (HPLC) CAS 165800-03-3View Details
Linezolid 99% (HPLC) CAS 165800-03-3View Details
165800-03-3 -
 LinezolidView Details
LinezolidView Details
165800-03-3 -
 Powder Linezolid -CAS No-165800-03-3View Details
Powder Linezolid -CAS No-165800-03-3View Details
165800-03-3 -
 Linezolid . .View Details
Linezolid . .View Details
165800-03-3
