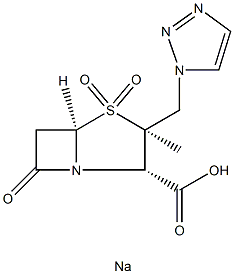
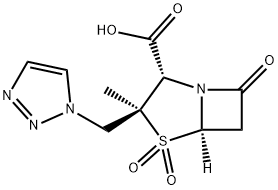
Tazobactam sodium
Synonym(s):[2S-(2α, 3β, 5α)]-3-Methyl-7-oxo-3(1H,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide;CL-298741;YTR-830H
- CAS NO.:89785-84-2
- Empirical Formula: C10H12N4NaO5S
- Molecular Weight: 323.28
- MDL number: MFCD00917472
- EINECS: 685-629-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-29 15:43:00
What is Tazobactam sodium?
Description
Tazobactam sodium is a new triazolylmethyl beta-lactamase inhibitor launched in combination with the antibiotic piperacillin as tazocilline. Tazobactam sodium is active against penicillinases and wide spectrum of beta-lactamases. The combination product tazobactdpiperacillin is effective against a broad range of both Gram-positive and -negative organisms and is indicated for the treatment of lower respiratory tract, urinary tract, intra-abdominal, biliary and skin and soft tissue infections. It is expected to compete against the combination product augmentin (amoxicillidclavulanate).
Chemical properties
White to off-white powder
Originator
Taiho (Japan)
The Uses of Tazobactam sodium
Tazobactam sodium is a beta-Lactamase inhibitor, which is used with penicillinase inhibition studies and beta-lactam antibiotics to enhance their effect. It serves as an antibacterial penicillin derivative, which inhibits the action of bacterial beta-lactamases.
The Uses of Tazobactam sodium
Lactamase inhibitor, used with ?lactam antibiotics to enhance their effect
The Uses of Tazobactam sodium
β-Lactamase inhibitor, used with β-lactam antibiotics to enhance their effect.
The Uses of Tazobactam sodium
A β-lactamase inhibitor and antibacterial agent
What are the applications of Application
Tazobactam sodium is a β-lactamase inhibitor and antibacterial agent
Definition
ChEBI: Tazobactam sodium is an organic sodium salt having tazobactam(1-) as the counterion; used in combination with ceftolozane sulfate for treatment of complicated intra-abdominal infections and complicated urinary tract infections. It has a role as an antimicrobial agent, an antiinfective agent and an EC 3.5.2.6 (beta-lactamase) inhibitor. It contains a tazobactam(1-).
Manufacturing Process
A known β-lactam type antibiotic (for example, benzhydryl 2-β-chloromethyl-
2-α-methylpenam-3-α-carboxylate) was used for synthesis of new penicillinic
derivatives.
A solution of 5.00 g of sodium azide in 53 ml of water was added to a solution
of benzhydryl 2-β-chloromethyl-2-α-methylpenam-3-α-carboxylate (5.13 g) in
dimethylformamide (155 ml). The mixture was stirred at room temperature
for 4 h. The resulting reaction mixture was poured into cooled water and the
mixture was extracted with ethyl acetate. The ethyl acetate layer was washed
with water, dried over magnesium sulfate and concentrated to provide 4.87 g
of the benzhydryl 2-β-azidomethyl-2-α-methylpenam-3-α-carboxylate as oil in
93% yield.
To a solution of benzhydryl 2-β-azidomethyl-2-α-methylpenam-3-α-
carboxylate (7.03 g) in a mixture of acetic acid (240 ml) and water (40 ml)
was added potassium permanganate (6.02 g) over a period of more than 1 h.
The mixture was stirred at room temperature for 2.5 h. The resulting reaction
mixture was diluted with ice water. The precipitate was collected by filtration,
and washed with water. The resulting product was dissolved in ethyl acetate
and the solution was washed with an aqueous solution of sodium hydrogen
carbonate and dried over magnesium sulfate. Concentration gave 5.48 g of
the benzhydryl 2-β-azidomethyl-2-α-methylpenam-3-α-carboxylate-1,1-dioxide
in 72% yield.
A 200 mg quantity of benzhydryl 2-β-azidomethyl-2-α-methylpenam-3-α-
carboxylate-1,1-dioxide was reacted with 10 ml of vinyl acetate in a sealed reactor at 100° to 110°C for 30 h. The reaction mixture was concentrated at
reduced pressure. The residue was crystallized with cooled chloroform. The
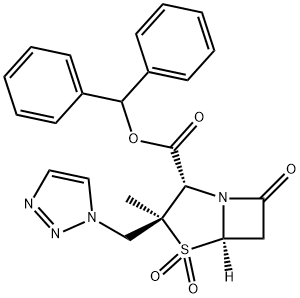
white crystals of benzhydryl 2-α-methyl-2-β-(1,2,3-triazol-1-yl)methylpenam-
3-α-carboxylate-1,1-dioxide have a melting point 206°-208°C, dec.
Hydrogenation was conducted in 10 ml of ethyl acetate and 10 ml of water at
room temperature for 30 min by using 45 mg of benzhydryl 2-α-methyl-2-β-
(1,2,3-triazol-1-yl)methylpenam-3-α-carboxylate-1,1-dioxide, 15 mg of 10%
palladium charcoal and 16 mg of sodium hydrogen carbonate. The aqueous
layer was separated from the reaction mixture and washed once with ethyl
acetate. The aqueous solution was then purified with an MCl gel, CHP-20P
(product of Mitsubishi Kasei Co., Ltd., Japan). After freeze-drying, there was
obtained an amorphous product of sodium 2-α-methyl-2-β-(1,2,3-triazol-1-
yl)methylpenam-3-α-carboxylate-1,1-dioxide with a melting point 170°C, dec.
brand name
Tazocilline
Therapeutic Function
Antibiotic
Properties of Tazobactam sodium
| Melting point: | 140-147°C |
| Boiling point: | 77℃ |
| Flash point: | >110°(230°F) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | powder |
| color | white |
| optical activity | [α]/D 144±4°, c = 0.1 in H2O |
| Water Solubility | Soluble in water.Soluble in water and methanol. |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 89785-84-2(CAS DataBase Reference) |
Safety information for Tazobactam sodium
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P271:Use only outdoors or in a well-ventilated area. |
Computed Descriptors for Tazobactam sodium
Tazobactam sodium manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



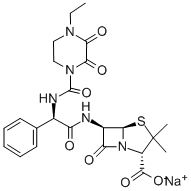
You may like
-
 Tazobactam sodium salt CAS 89785-84-2View Details
Tazobactam sodium salt CAS 89785-84-2View Details
89785-84-2 -
 Tazobactam SodiumView Details
Tazobactam SodiumView Details
89785-84-2 -
 Tazobactam SodiumView Details
Tazobactam SodiumView Details
89785-84-2 -
 Tazobactam SodiumView Details
Tazobactam SodiumView Details
89785-84-2 -
 Tazobactam SodiumView Details
Tazobactam SodiumView Details
89785-84-2 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
