Tazarotene
Synonym(s):6-[(3,4-Dihydro-4,4-dimethyl-2H-1-benzothiopyran-6-yl)ethynyl]-3-pyridinecarboxylic acid ethyl ester;AGN-190168;ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)ethynyl] nicotinate
- CAS NO.:118292-40-3
- Empirical Formula: C21H21NO2S
- Molecular Weight: 351.46
- MDL number: MFCD00867628
- EINECS: 601-516-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
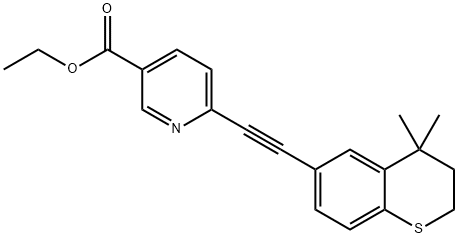
What is Tazarotene?
Absorption
Minimal systemic absorption of tazarotene occurs due to its rapid metabolism in the skin to the active metabolite, tazarotenic acid, which can be systemically absorbed and further metabolized. Gender had no influence on the systemic bioavailability of tazarotenic acid.
Toxicity
Excessive topical use may lead to marked redness, peeling, or discomfort. Oral ingestion of the drug may affect liver function causing hypertriglyceridemia. Other symptoms may include conjunctival irritation, hair loss, headache, edema, fatigue, dermatitis, nausea, and visual disturbances. Oral administration of this material to rats and rabbits at doses of 0.20 mg/kg/day (rabbits) and 0.25 mg/kg/day (rats) resulted in developmental toxicity. A no effect level of 0.05 mg/kg/day was established. Similar teratogenic effects have been reported for other retinoid compounds.
Chemical properties
White Solid
Originator
Avage,Allergan
The Uses of Tazarotene
Tazarotene is a prescription topical retinoid sold as a cream or gel. This medication is approved for treatment of psoriasis, acne, and sun damaged skin (photodamage). It is commonly sold in two concentrations: 0.05% and 0.1%. In addition to tretinoin, wh
The Uses of Tazarotene
An acetylenic retinoid prodrug converted to the active metabolite, Tazarotenic acid, with selective affinity for retinoic acid receptors RAR?and RAR. Antiacne; antipsoriatic. Used in treatment of photodamaged skin
The Uses of Tazarotene
Used to treat psoriasis, acne and sun damaged skin.
Indications
Used to treat psoriasis, acne and sun damaged skin (photodamage).
Background
Tazarotene, commonly marketed as Tazorac?, Avage?, and Zorac?, is member of the acetylenic class of retinoids. It is a prodrug that is found in topical formulations used in the treatment of various conditons, such as psoriasis, acne, and sun damaged skin (photodamage).
Definition
ChEBI: The ethyl ester of tazarotenic acid. A prodrug for tazarotenic acid, it is used for the treatment of psoriasis, acne, and sun-damaged skin.
Indications
Like other retinoids, tazarotene (Tazorac) acts by binding to RARs and altering gene expression. Tazarotene appears to be particularly selective for the retinoid receptors RAR-β and RAR-γ, but the clinical significance of this observation is unknown.
Manufacturing Process
Methods of preparation of the ethyl 6-[2-(4,4-dimethylthiochroman-6- yl)ethynyl]nicotinate
1.Reaction vessels used in this procedure were flame dried under vacuum and all operations carried out in an oxygen-free, argon or nitrogen atmosphere. To a solution of 465.7 mg (2.3019 mmol) of 4,4-dimethyl-6- ethynyl-thiochroman in 4 ml of dry tetrahydrofuran at 0°C was added dropwise 1.5 ml of 1.6 M (2.4 mmol) n-butyl lithium in hexane. This was stirred at 0°C for 10 min and at room temperature for 10 min, cooled again to 0°C and then treated with a solution of 330 mg (2.4215 mmol) of fused ZnCl2 in 4 ml dry tetrahydrofuran using a double ended needle. Thereafter the solution was stirred at 0°C for 30 min, then at room temperature for 10 min. A solution of 426.3 mg (2.2967 mmol) of ethyl 6-chloronicotinoate in 4 ml dry tetrahydrofuran was transferred by double ended needle into a suspension of 430 mg (0.37 mmol) of tetrakistriphenylphosphine palladium in 4 ml dry tetrahydrofuran and stirred at room temperature for 10 min, then treated by double ended needle with the solution of the alkynylzinc prepared above. This mixture was stirred at room temperature for 18 h, then quenched with 100 ml water. Product was recovered by extraction with 3 times 75 ml ether. Ether fractions were combined and washed with saturated NaCl solutions and dried. Solvent was removed in vacuo and the residue purified by flash chromatography (silica; 5% ethyl acetate in hexane) followed by HPLC (Whatman Partisil M-9 10/50; 4% ethyl acetate in hexane) to give the ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)ethynyl]nicotinate.
2.A solution of 15.4 g (76.2 mmol) of 4,4-dimethyl-6-ethynylthiochroman and 14.0 g (75.5 mmol) of ethyl-6-chloronicotinate in 35 ml of freshly distilled triethylamine was degassed and then treated under nitrogen with a finely powdered mixture of 1 g (5.25 mmol) of high purity cuprous iodide and 2 g (2.85 mmol) of bis(triphenylphosphine) palladium (II) chloride. The mixture was heated under nitrogen at 55°C for 20 h and then cooled to room temperature. The triethylamine was then removed under vacuum and the residue was diluted with 200 ml of a 1:4 mixture of ethyl acetate and hexanes. This mixture was filtered through silica and the filtrate concentrated in vacuo. The resultant residue was purified by flash chromatography (silica gel; 15% ethyl acetate in hexanes) and recrystallized from a mixture of ethyl acetate and hexanes to give the ethyl 6-[2-(4,4-dimethylthiochroman-6- yl)ethynyl]nicotinate as a pale yellow solid.
brand name
Avage (Allergan); Tazorac (Allergan).
Therapeutic Function
Keratolytic
Biochem/physiol Actions
Tazarotene induces the expression of tazarotene-induced gene 3 (TIG3), a tumor suppressor gene. It is a prodrug of tazarotenic acid, which specifically activates RARb and RARg, only weakly activates RARa, and is inactive at retinoid X receptors (RXRs). In psoriasis, tazarotene normalizes abnormal keratinocyte differentiation and reduces their hyperproliferation.
Pharmacokinetics
Following topical application, tazarotene undergoes esterase hydrolysis to form its active metabolite, tazarotenic acid. When treating acne tazarotene may be taken in conjunction with an oral antibiotic. Tazarotene has been shown in peer-reviewed double blinded studies to reduce: mottling and hyperpigmentation, sallowness, fine wrinkling and coarse wrinkling in sun damaged skin. Histological studies have shown that long term (greater than 1 year) use of Tazarotene is associated with a significant reduction in atypical melanocytes and keratocytes - cells considered to be precursors of skin cancer. Some studies have shown long term use of Tazarotene to be associated with increased collagen production and better organization of skin collagen bundles.
Clinical Use
In the United States, tazarotene has been approved for topical treatment of psoriasis (involving up to 20% body surface area) and mild to moderate facial acne. Application site burning, stinging, and desquamation are common side effects, especially with acne. Tazarotene is contraindicated in women who are pregnant.
Side Effects
Tazorac is a category X drug and must be avoided in pregnancy. This drug can be irritating and should be avoided in patients with sensitive skin or seborrheic dermatitis.
Metabolism
Undergoes esterase hydrolysis in skin to form its active metabolite, tazarotenic acid. Tazarotenic acid is further metabolized in skin and, after systemic absorption, hepatically metabolized to sulfoxides, sulfones, and other polar products for elimination.
Structure and conformation
Retinoid prodrug, converted to active form, the carboxylic acid of tazarotene by rapid de-esterification. This drug binds to all three retinoic acid receptors with some increased affinity for the β and γ receptors.
Properties of Tazarotene
| Melting point: | 95-98°C |
| Boiling point: | 499.8±45.0 °C(Predicted) |
| Density | 1.22±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >15mg/mL |
| form | powder |
| pka | 0.04±0.29(Predicted) |
| color | white to very faintly yellow |
| Merck | 14,9081 |
| CAS DataBase Reference | 118292-40-3(CAS DataBase Reference) |
Safety information for Tazarotene
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Tazarotene
Tazarotene manufacturer
BDR Pharmaceuticals International Pvt Ltd
Maithri Drugs Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 TAZAROTENE 99%View Details
TAZAROTENE 99%View Details -
 Tazarotene 98%View Details
Tazarotene 98%View Details
118292-40-3 -
 Tazarotene, ≥98% (HPLC) CAS 118292-40-3View Details
Tazarotene, ≥98% (HPLC) CAS 118292-40-3View Details
118292-40-3 -
 Tazarotene CAS 118292-40-3View Details
Tazarotene CAS 118292-40-3View Details
118292-40-3 -
 Tazarotene 99%View Details
Tazarotene 99%View Details
118292-40-3 -
 Tazarotene 118292-40-3 98%View Details
Tazarotene 118292-40-3 98%View Details
118292-40-3 -
 Tazarotene 98% CAS 118292-40-3View Details
Tazarotene 98% CAS 118292-40-3View Details
118292-40-3 -
 Tazarotene CAS 118292-40-3View Details
Tazarotene CAS 118292-40-3View Details
118292-40-3